A cumulene is a compound with three adjacent double bonds. Draw an orbital picture of a cumulene.
Question:
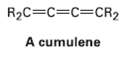
A cumulene is a compound with three adjacent double bonds. Draw an orbital picture of a cumulene. What kind of hybridization do the two central carbon atoms have? What is the geometric relationship of the sub-stituents on one end to the sub-stituents on the other end? What kind of isomerism is possible? Make a model to help see theanswer.
Transcribed Image Text:
R2C=C=C=CR2 A cumulene
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (8 reviews)
sp2 R R spsp o bond a bonds sp sp o bond bonds sp spsp o bond sp R3 R4 This simple...View the full answer

Answered By

ANDREW KIPRUTO
Academic Writing Expert
I have over 7 years of research and application experience. I am trained and licensed to provide expertise in IT information, computer sciences related topics and other units like chemistry, Business, law, biology, biochemistry, and genetics. I'm a network and IT admin with +8 years of experience in all kind of environments.
I can help you in the following areas:
Networking
- Ethernet, Wireless Airmax and 802.11, fiber networks on GPON/GEPON and WDM
- Protocols and IP Services: VLANs, LACP, ACLs, VPNs, OSPF, BGP, RADIUS, PPPoE, DNS, Proxies, SNMP
- Vendors: MikroTik, Ubiquiti, Cisco, Juniper, HP, Dell, DrayTek, SMC, Zyxel, Furukawa Electric, and many more
- Monitoring Systems: PRTG, Zabbix, Whatsup Gold, TheDude, RRDtoo
Always available for new projects! Contact me for any inquiries
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Draw an orbital picture of furan to show how the molecule isaromatic. Furan :O:
-
Draw an orbital picture of thiazole. Assume that both the nitrogen and sulfur atoms are sp2-hyhridized, and show the orbitals that the lone pairs occupy.
-
What kind of hybridization do you expect for each carbon atom in the followingmolecules? (a) Propane, CH3CH2CH3 (b) 2-Methylpropene, CH CH3C=CH2 (c) 1-Butene 3 yne, H2C=CH-C=CH (d) Acetic acid, CHC
-
The balance sheet for the Heir Jordan Corporation follows. Based on this information and the income statement in the previous problem, supply the missing information using the percentage of sales...
-
You are the Director of the Computer Center for Gaillard College and responsible for scheduling the staffing of the center, which is open from 8 a.m. until midnight. You have monitored the usage of...
-
Derive the expressions for the velocity distribution and for the pressure drop for a Newtonian fluid in fully developed laminar flow in the annular space between two horizontal, concentric pipes....
-
The Tinchers signed a contract to sell land to Creasy. The contract specified that the sales transaction was to be completed in 90 days. At the end of the 90 days, Creasy requested an extension of...
-
Tarheel Furniture Company is planning to establish a wholly owned subsidiary to manufacture upholstery fabrics. Tarheel expects to earn $1 million after taxes on the venture during the first year....
-
Examples of committed fixed costs include all of the following except: Examples of committed fixed costs include all of the following except
-
Lakeway Manufacturing Co. manufactures and sells household cleaning products. The company's research department has developed a new cleaner for which a standard cost must be determined. The new...
-
Terminal alkynes react with Br2 and water to yield bromo ketones. For example: Propose a mechanism for the reaction. To what reaction of alkenes is the processanalogous? -CECH Br2, H20 CH2Br
-
Reaction of acetone with D 3 O + yields hexadeuterioacetone. That is, all the hydrogen?s in acetone are exchanged for deuterium. Review the mechanism of mercuric ion?catalyzed alkyne hydration, arid...
-
The following are weekly sales data, in thousands of units, for microcomputer disks: 57, 58, 60, 54, 56, 53, 55, 59, 62, 57, 50, 48, 52, 55, 58, 61 Use w = 0.3 and w = 0.8 to produce an exponential...
-
Question (4) seen, 20 vehicles/km moving at 100 km/h and 30 vehicles/km traveling at 120 km/h. Two successive videos showing stationary traffic on the road were examined. Two groups of platoons were...
-
?In civil engineering, what is the main use of a slump test in concrete technology?
-
Explain the process of compression resin transfer molding(CRTM)?in composite manufacturing. What are the benefits of using CRTM for producing composite structures?
-
Explore the role of post-occupancy evaluation in commercial and industrial architecture. How do architects use feedback from building users to improve future designs?
-
Discuss the principles of geotechnical engineering in slope stability analysis. How can engineers assess slope stability, mitigate landslide risks, and design effective stabilization measures to...
-
The special event industry can be grouped into several smaller classifications including corporate events, association events, charity balls and fund-raising events, social functions, fairs and...
-
Which, if any, of the dichloroethene molecules drawn in Data Table II (3.) (4.) and (5.) are geometric isomers? A. B. C. D. cis-1,2-dichloroethene and trans-1,2-dichloroethene...
-
Determine whether each redox reaction is spontaneous. (a) Fe(s) + Mg2+ (aq) (b) Fe(s) + Pb+ (aq) Fe2+ (aq) + Mg(s) 2+ Fe+ (aq) + Pb(s)
-
(a) Draw the structure of cis-CH3-CH=CH-CH2CH3 showing the pi bond with its proper geometry. (b) Circle the six coplanar atoms in this compound. (c) Draw the trans isomer, and circle the coplanar...
-
In pent-2-yne (CH3CCCH2CH3) there are four atoms in a straight line. Use dashed lines and wedges to draw a threedimensional representation of this molecule, and circle the four atoms that are in a...
-
Which of the following compounds show cis-trans isomerism? Draw the cis and trans isomers of the ones that do. (a) CH3CH=CHCH3 (b) CH3-C¡C-CH3 (c) CH2=C(CH3)2 (d) (e) (f) CH3-CH=N-CH3...
-
Suppose a bond has a modified duration of 4. By approximately how much will the bonds value change if interest rates: a. Increase by 50 basis points b. Decrease by 150 basis points c. Increase by 10...
-
You have just been hired as a new management trainee by Earrings Unlimited, a distributor of earrings to various retail outlets located in shopping malls across the country. In the past, the company...
-
Difference between Operating Leverage and Financial Leverage

Study smarter with the SolutionInn App


