What kind of hybridization do you expect for each carbon atom in the followingmolecules? (a) Propane, CH3CH2CH3
Question:
What kind of hybridization do you expect for each carbon atom in the followingmolecules?
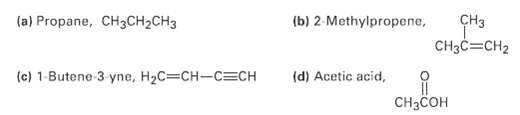
Transcribed Image Text:
(a) Propane, CH3CH2CH3 (b) 2-Methylpropene, CHз CH3C=CH2 (c) 1-Butene 3 yne, H2C=CH-C=CH (d) Acetic acid, CHзCсон
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 52% (19 reviews)
a sp3 sp3 sp3 CH3C...View the full answer

Answered By

Muhammad Haroon
More than 3 years experience in teaching undergraduate and graduate level courses which includes Object Oriented Programming, Data Structures, Algorithms, Database Systems, Theory of Automata, Theory of Computation, Database Administration, Web Technologies etc.
5.00+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
What kind of hydribization do you expect for each carbon atom in the followingmolecules? CH- (b) H. C-H (a) 1 "CH-CH CH2 C=C H2N H. Procaine Vitamin C (ascorbic acid)
-
What hybridization do you expect for the atom indicated in red in each of the following species? (a) CH3CO2- (b) PH4+ (c) AlF3 (d) H2C==CH-CH2+
-
What dividends do you expect for Goodman Industries stock over the next 3 years if you expect the dividend to grow at the rate of 5% per year for the next 3 years? In other words, calculate D1, D2,...
-
Would individual mandates for health insurance be more or less burdensome to the poor than employer mandates? Would lower-income groups be wise to favor one plan over the other?
-
Art Funkel started his incorporated medical practice on June 1, 2016. He immediately made an Selection for the corporation. Art would like the corporation to adopt a tax year ending May 31 so that a...
-
A 0.180-kg cube of ice (frozen water) is floating in glycerine. The gylcerine is in a tall cylinder that has inside radius 3.50 cm. The level of the glycerine is well below the top of the cylinder....
-
What do you think of Ubisoft's approach?
-
Consider the following program: Note that the scheduler in a uniprocessor system would implement pseudo parallel execution of these two concurrent processes by interleaving their instructions,...
-
Which of the following would be considered as indirect labour?O a . A lawyer in a legal firm b . A stores assistant in a factory storeroomO c . Plasterers in a building company d . Assembly workers
-
Magnificent Modems, Inc., makes modem cards that are used in notebook computers. The company completed the following transactions during 2010. All purchases and sales were made with cash. 1. Acquired...
-
Convert the following molecular formulas into line-bond structures that are sonsistent with valence rules: (a) C3H8 (b) CH5N (c) C2H6O (2 possibilities) (d) C3H7Br (2 possibilities) (e) C2H4O (3...
-
What is the shape of benzene, and what hydribization do you expect for eachcarbon? C=c H-C - Benzene C-C I.
-
Explain what is meant by market stabilization.
-
Popcorn company is expected to pay $1 dividend per share at the end of this year, $1.50 dividend per share at the end of year 2, $2 dividend per share at the end of year 3, and $2.50 dividend per...
-
Increased spending for COVID economic relief is an important issue for many struggling in the current economy. A specific policy to combat this issue is put forward and it is found that 78% of...
-
James worked a total of 186 hours for the month of June 2020. His rate per hour is working hours of the 450 per hour. Overtime premium is 30%. The company is 8 hours a day. The company's regular...
-
Question Researchers collected a simple random sample of 36 children who had been identified as gifted in a large city. The following histograms show the distributions of the IQ scores of mothers and...
-
Shown below is activity for one of the products of Denver Office Equipment: January 1 balance, 700 units @ $55 per unit $38,500 Purchases: January 10: 700 units @ $60 per unit January 20: 1,100 units...
-
Explain the VFM concept and describe the link between economy, efficiency and effectiveness.
-
Diamond Walker sells homemade knit scarves for $25 each at local craft shows. Her contribution margin ratio is 60%. Currently, the craft show entrance fees cost Diamond $1,500 per year. The craft...
-
Calculate the vapor pressure of n-butane as a function of temperature using the Peng-Robinson equation of state. Compare your results with (a) Literature values (b) Predictions using the...
-
Explain whether each pair of models represents isomers or the same compound. (All represent compounds with the formula C7H16.) Draw structures for each compound represented by the models.
-
Explain whether each pair of models represent isomers or the same compound. Draw structures for each compound represented by the models.
-
The following models represent three isomers of C6H4Cl2. Explain which of these compounds does not have a dipole moment.
-
why would an auditor want to complete dual-purpose tests? what procedure can be put into place to help prevent fraud? List 4 procedures.
-
Based on the following information, calculate sustainable growth rate for Groot, Inc.: Profit margin= 7.1% Total asset turnover = 1.90 Total debt ratio = .45 Payout ratio = 20% What is the ROA here?
-
Consider the following: a call option on a stock has strike price $100, premium of $5 and the current price of the underlying stock is $100. If you buy the call option today, what is your holding...

Study smarter with the SolutionInn App


