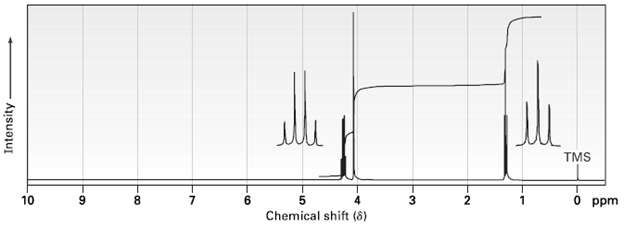
The compound whose 1 H NMR spectrum is shown has the molecular formula C 4 H 7
Question:
The compound whose 1H NMR spectrum is shown has the molecular formula C4H7O2C1 and has an infrared absorption peak at 1740 cm?1. Propose a structure.
Transcribed Image Text:
TMS 10 O ppm Chemical shift (8) Intensity
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 70% (10 reviews)
Possible structures for C4HClO are CH3CHCOCHCl and CICHCOCHCH3 Chemical shift data can distingu...View the full answer

Answered By

Sheikh Muhammad Ibrahim
During the course of my study, I have worked as a private tutor. I have taught Maths and Physics to O'Level and A'Level students, as well as I have also taught basic engineering courses to my juniors in the university. Engineering intrigues me alot because it a world full of ideas. I have passionately taught students and this made me learn alot. Teaching algebra and basic calculus, from the very basics of it made me very patient. Therefore, I know many tricks to make your work easier for you. I believe that every student has a potential to work himself. I am just here to polish your skills. I am a bright student in my university. My juniors are always happy from me because I help in their assignments and they are never late.
4.90+
14+ Reviews
24+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
The ketone whose 1H NMR spectrum is shown here was obtained as the product of an acetoacetic ester synthesis. What alkyl halide was used in the synthesis? 10 (ppm)
-
The following 1H NMR spectrum is that of an alcohol, C8H10O. Propose a structure. TMS 6. Chemical shift (8) 3 O ppm 10 8. Intensity
-
An infrared absorption spectrum of an organic compound is shown in the following figure. Use the characteristic group frequencies listed in Section 19.5 to decide whether this compound is more likely...
-
Estimates have been presented to Holly Farms, which is considering two environmental chambers for a project that will detail laboratory confirmations of on-line bacteria tests in chicken meat for the...
-
Some contracts, such as those between municipalities and highway construction firms, are extremely long with terms spelled out in minute detail. Others, such as those between consulting firms and...
-
Let A and B be events with P(A) = 0.5 and P(A B c ) = 0.4. For what value of P(B) will A and B be independent?
-
Name the major gaming entertainment hotels in Las Vegas.
-
Does this case indicate that JPMorgan and the federal government were in a collaborative partnership or working at arms length? Why do you think so?
-
answer urgently Julie Ellis, Sara Lake, and Dan Madden have capital balances of $ 54,000, S 82,000, and $ 36,000, respectively, and their profit ratios are 4:2:4. Required: Do not use commas when...
-
1. Identify the different power issues going on in the case. What types of power do the different parties have? Explain. 2. How are individuals reacting to their power or lack thereof? 3. What types...
-
Propose structures for compounds that fit the following 1H NMR data: (a) C5H10O 0.95 (6 H, doublet, J = 7 Hz) 2.10 (3 H, singlet) 2.43 (1 H, multiplet) (b) C3H5Br 2.32 (3 H, singlet) 5.35 (1 H,...
-
Propose structures for compounds that fit the following 1 H NMR data: (a) C 4 H 6 Cl 2 2.18 (3 H, singlet) 4.16 (2 H, doublet, J = 7 Hz) 5.71 (1 H, triplet, I = 7 Hz) (b) C 10 H 14 1.30 (9 H,...
-
List the elements of the emergency management program.
-
Based on a survey, assume that 42% of consumers are comfortable having drones deliver their purchases. Suppose that we want to find the probability that when six consumers are randomly selected,...
-
What is the social location that determines this speech community? Is it determined by race, class, gender, sexuality, or some other social location? What makes this speech community unique? What are...
-
Write a program named SumOfNumberOfSquares.java that prompts user to enter a number of integers and calculates the sum of their squares. The following is a sample run. The green fonts represent user...
-
6.4 Charles Augustin de Coulomb was a French physicist who is best known for formulating the law that calculates the force between two electric charges. To honor Coulomb, the unit of electric charge...
-
What amount of cash payments to suppliers will be reported by Indigo Company for the year ended December 31, 2024?
-
Data Analytics Application: Identify the work environment variables from Samouels employee survey. Identify the six statements covering coworkers and supervision. Use statistical software to...
-
The area of square PQRS is 100 ft2, and A, B, C, and D are the midpoints of the sides. Find the area of square ABCD. B A
-
Scuba divers breathing air at increased pressure can suffer from nitrogen narcosisa condition resembling drunkennesswhen the partial pressure of nitrogen exceeds about 4 atm. What property of...
-
Predict the products of the following proposed Diels-Alder reactions. (a) (b) (c) (d) (e) (f) CHO C-C-IC-C CN NC CN O + OCHs CN CH,O CN
-
What dienes and dienophiles would react to give the following Diels-Alder products? (a) (b) (c) (d) (e) (f) C-CH CH3O CN C OCH C--OCH, CN CN CH,O CN H O
-
Predict the major product for each proposed Diels-Alder reaction. Include stereochemistry where appropriate. (a) (b) (c) Ph 0 Ph
-
The following are the information of Chun Equipment Company for Year 2 . ( Hint: Some of the items will not appear on either statement, and ending retained earnings must be calculated. ) Salaries...
-
Alta Ski Company's inventory records contained the following information regarding its latest ski model. The company uses a periodic inventory system. Beginning inventory, January 1, 2018 1,250 units...
-
Fibertech GmbH is a distributor of outdoors technical clothing. The company outsources the production of clothing to external manufacturers in Bangladesh and sells the clothing under its own brands....

Study smarter with the SolutionInn App


