The experiment in Figure 16-8 required 5.32 mA for 964 s for complete reaction of a 5.00
Question:
The experiment in Figure 16-8 required 5.32 mA for 964 s for complete reaction of a 5.00 mL aliquot of unknown cyclohexene solution.
(a) How many moles of electrons passed through the cell?
(b) How many moles of cyclohexene reacted?
(c) What was the molarity of cyclohexene in the unknown?
Figure 16-8
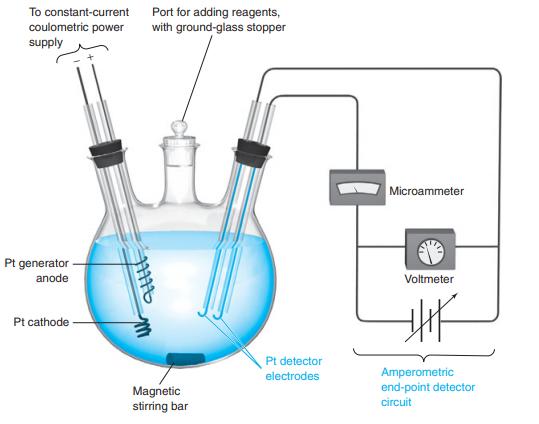
Transcribed Image Text:
To constant-current coulometric power supply Port for adding reagents, with ground-glass stopper Microammeter Pt generator anode Voltmeter Pt cathode Pt detector Amperometric end-point detector electrodes Magnetic stirring bar circuit
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 58% (12 reviews)
a mole b One mol e reacts with mol Br 2 w...View the full answer

Answered By

Asim farooq
I have done MS finance and expertise in the field of Accounting, finance, cost accounting, security analysis and portfolio management and management, MS office is at my fingertips, I want my client to take advantage of my practical knowledge. I have been mentoring my client on a freelancer website from last two years, Currently I am working in Telecom company as a financial analyst and before that working as an accountant with Pepsi for one year. I also join a nonprofit organization as a finance assistant to my job duties are making payment to client after tax calculation, I have started my professional career from teaching I was teaching to a master's level student for two years in the evening.
My Expert Service
Financial accounting, Financial management, Cost accounting, Human resource management, Business communication and report writing. Financial accounting : • Journal entries • Financial statements including balance sheet, Profit & Loss account, Cash flow statement • Adjustment entries • Ratio analysis • Accounting concepts • Single entry accounting • Double entry accounting • Bills of exchange • Bank reconciliation statements Cost accounting : • Budgeting • Job order costing • Process costing • Cost of goods sold Financial management : • Capital budgeting • Net Present Value (NPV) • Internal Rate of Return (IRR) • Payback period • Discounted cash flows • Financial analysis • Capital assets pricing model • Simple interest, Compound interest & annuities
4.40+
65+ Reviews
86+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Chemical Engineering questions
-
The active ingredients in an antacid tablet contained only calcium carbonate and magnesium carbonate. Complete reaction of a sample of the active ingredients required 41.33 mL of 0.08750 M...
-
Explain how the amperometric end-point detector in Figure 16-8 operates. Figure 16-8 To constant-current coulometric power supply Port for adding reagents, with ground-glass stopper Microammeter Pt...
-
Does the MO energy diagram of cyclooctatetraene (Figure 16-8) appear to be a particularly stable or unstable configuration? Explain. In Figure 16.8 nonbonding line cyclobutadiene cyclooctatetraene...
-
Modify Lookup to make a program LookupAndPut that allows put operations to be specified on standard input. Use the convention that a plus sign indicates that the next two strings typed are the...
-
At the beginning of 2012, Mazzaro Company acquired equipment costing $120,000. It was estimated that this equipment would have a useful life of 6 years and a salvage value of $12,000 at that time....
-
Neal is adjudged mentally incompetent, and a guardian is appointed. Neal later signs an investment contract with Delfina. This contract is a. valid. b. voidable. c. void. d. none of the above.
-
Go to www.acq.osd.mil/evm and explore the various links and screens. What does the size and diversity of this site tell you about the acceptance and use of earned value in organizations today?
-
During normal business hours on the east coast, calls to the toll-free reservation number of the Nite Time Inn arrive at a rate of 5 per minute. It has been determined that the number of calls per...
-
i need help with d and e exhibit 3.1 i dont know what else would be incomplete $69,000 bill from her accountant for consulting services related to her small business. Heese can January 30 of next...
-
Digital Controls, Inc. (DCI), manufactures two models of a radar gun used by police to monitor the speed of automobiles. Model A has an accuracy of plus or minus 1 mile per hour, whereas the smaller...
-
The sensitivity of a coulometer is governed by the delivery of its minimum current for its minimum time. Suppose that 5 mA can be delivered for 0.1 s. (a) How many moles of electrons are delivered by...
-
H 2 S(aq) can be analyzed by titration with coulometrically generated I 2 . H 2 S + I 2 + S(s) + 2H + + 2I - To 50.00 mL of sample were added 4 g of KI. Electrolysis required 812 s at 52.6 mA....
-
How would the grapevine play a role in the ways communication happens after Shopify adopted its less frequent meeting culture?
-
What is a transistor, and what are its types?
-
Discuss the emerging role of nanotechnology in electrical engineering, focusing on its applications in enhancing electrical components like batteries, supercapacitors, and sensors.Explore the...
-
1. As resistors are added in parallel to an existing circuit, what happens to the voltage drop measured across each resistor? 2. In the circuit shown on the right, which path (left or right) will...
-
What is the purpose of project feasibility?
-
Question 6.10 Current and deferred tax worksheets and tax entries From the hip Ltd?s statement of profit or loss for the year ended 30 June 2007 and extracts from its statements of financial position...
-
Antibonding molecular orbitals can be used to make bonds to other atoms in a molecule. For example, metal atoms can use appropriate d orbitals to overlap with the *2p orbitals of the carbon monoxide...
-
Methyl isocyanate, CH3NCO, was made infamous in 1984 when an accidental leakage of this compound from a storage tank in Bhopal, India, resulted in the deaths of about 3,800 people and severe and...
-
(a) Methane (CH4) and the perchlorate ion are both described as tetrahedral. What does this indicate about their bond angles? (b) The NH3 molecule is trigonal pyramidal, while BF3 is trigonal planar....
-
The predetermined overhead rate is usually calculated Group of answer choices At the end of each year At the beginning of each month At the beginning of the year At the end of the month
-
ajax county collects property taxes for the cities within the county, Ajax county collected 1000 from citizens in Beatty city that belong to Beatty city what would be the appropriate entries for ajax...
-
Assume that gasoline costs $ 3 . 2 0 per gallon and you plan to keep either car for six years. How many miles per year would you need to drive to make the decision to buy the hybrid worthwhile,...

Study smarter with the SolutionInn App


