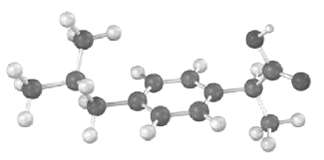
The following model is a representation of ibuprofen, a common over-the-counter pain reliever. Indicate the positions of
Question:
The following model is a representation of ibuprofen, a common over-the-counter pain reliever. Indicate the positions of the multiple bonds, and draw a skeletal structure (gray = C, red = O, ivory =H).
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 76% (17 reviews)

Answered By

ANDREW KIPRUTO
Academic Writing Expert
I have over 7 years of research and application experience. I am trained and licensed to provide expertise in IT information, computer sciences related topics and other units like chemistry, Business, law, biology, biochemistry, and genetics. I'm a network and IT admin with +8 years of experience in all kind of environments.
I can help you in the following areas:
Networking
- Ethernet, Wireless Airmax and 802.11, fiber networks on GPON/GEPON and WDM
- Protocols and IP Services: VLANs, LACP, ACLs, VPNs, OSPF, BGP, RADIUS, PPPoE, DNS, Proxies, SNMP
- Vendors: MikroTik, Ubiquiti, Cisco, Juniper, HP, Dell, DrayTek, SMC, Zyxel, Furukawa Electric, and many more
- Monitoring Systems: PRTG, Zabbix, Whatsup Gold, TheDude, RRDtoo
Always available for new projects! Contact me for any inquiries
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
The following model is a representation of asparatame, C14H18N2O5, known commercially as NutraSweet. Only the connection between atoms is shown; multiple bonds are not indicated. Complete the...
-
The following model is a representation of acetaminophen, a pain reliever sold in drugstores as Tylenol. Identify the hydribization of each carbon atom in acetaminophen, and tell which atoms have...
-
The following molecular model is a representation of para-aminobenzoic acid (PABA), the active ingredient in many sunscreens. Indicate the positions of the multiple bonds. And draw a skeletal...
-
Courts are more willing to find misrepresentation if the defendant has a fiduciary relationship with the plaintiff than if a transaction occurs at arms length between the parties. True False
-
Reba, a calendar year taxpayer, owns an office building that she uses in her business. The building is involuntarily converted on November 15, 2016. On January 5, 2017, Reba receives enough proceeds...
-
Explain how a consumer can display signs of purchase momentum?
-
Do those who vote differ from those who dont vote with regard to how much they trust the government? Using ANES data, run a t -test comparing those who voted in the 2012 election with those who didnt...
-
Low Carb Diet Supplement Inc. has two divisions. Division A has a profit of $156,000 on sales of $2,010,000. Division B is only able to make $28,800 on sales of $329,000. Based on the profit margins...
-
The accounting manager at Essex Trucking Co. records the following transactions for March which have been journalized and posted to the proper accounts. Mar. 1 The business received $8,000 cash and...
-
Oil Co is building a refinery to produce four products: diesel, gasoline, lubricants, and jet fuel. The minimum demand (in bbl/day) for each of these products is 14,000,30,000, 10,000, and 8,000,...
-
Fill in the multiple bonds in the following model of naphthalene, C10H8 (gray = C, ivory =H). How many resonance structures does naphthalenehave?
-
Cis-1, 2-Dichloroethylene and trans-dichloroethylene are isomers, compounds with the same formula but different chemical structures. Look at the following electrostatic potential maps, and tell...
-
It is 165 cm from your eyes to your toes. Youre standing 200 cm in front of a tall mirror. How far is it from your eyes to the image of your toes?
-
Nequired information Exercise 5-17 (Static) Notes receivable-interest accrual and collection LO 5-6 (The following information applies to the questions displayed below) Agrico Incorporated accepted...
-
Case 14-3 Sarin Pharmaceuticals Ltd. Alan Mannik, director of procurement for the Sarin Phar- maceuticals Ltd. (Sarin) Animal Health Division plant in Vancouver, British Columbia, was planning for...
-
CL727 LEGAL ANALYSIS AND WRITING Module 11 Assignment: Brief Answer, Analysis, and Conclusion This assignment will be due in Module 11. Your assignment is to write the Brief Answer, Analysis, and...
-
Question 11 (0.5 points) l) Listen } As a drug manufacturer, you expect your latest wonder drug to lower cholesterol. It has been successful with a limited group of participants so far, so you have...
-
Redfern Audio produces audio equipment including headphones. At the Campus Facility, it produces two wireless models, Standard and Enhanced, which differ both in the materials and components used and...
-
Which practical reason that organizations need to change is inappropriate? a. Increasing competition means the flexible, ever-changing organization is now the norm. b. More participation by employees...
-
Read Case Study Google: Dont Be Evil Unless and answer the following: Why do you think Google was adamant about not wanting to supply information requested by the government concerning the Child...
-
a. Show that the intrinsic stability analysis for fluid equilibrium at constant temperature and volume leads to the single condition that b. Show that intrinsic stability analysis for fluid...
-
Explain which compound has the higher boiling point: (a) Octane or nonane (b) Nonane or 3-nonene (c) 1-Nonyne or 1-nonanol (d) Trim ethylamine or propylamine (e) Cyclopentanol or diethyl ether (f)...
-
Chloroform, CHCl3, is a common solvent in the organic laboratory. It is not miscible with water, so a mixture of these two solvents forms two layers, which solvent do you expect to form the lower...
-
Predict which of these compounds has the higher solubility in water: (a) 1-Butanol or 1-chlorobutane (b) 1-Butanol or 1-hexanol (c) Pentane or diethyl amine
-
Assume the market portfolio of common stocks earned 14.1 percent in one year while U.S. Treasury bills earned 4.4 percent and inflation averaged 4.6 percent. What was the market risk premium?
-
A bank quotes you an interest rate of 9 . 5 % per annum with quarterly compounding. What is the equivalent rate with continuous compounding?
-
Using automation tools leads to dislike 5 9 . How do the mechanical properties of woven fabric composites compare to those of unidirectional composites? Provide examples of applications for each type.

Study smarter with the SolutionInn App


