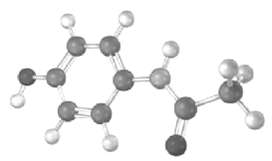
The following model is a representation of acetaminophen, a pain reliever sold in drugstores as Tylenol. Identify
Question:
The following model is a representation of acetaminophen, a pain reliever sold in drugstores as Tylenol. Identify the hydribization of each carbon atom in acetaminophen, and tell which atoms have lone pairs of electrons (gray = C, red = O, blue =H).
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 81% (11 reviews)
H H H CC 11 H CCsp 11 H O H Acetamino...View the full answer

Answered By

Munir Ahmed Jakhro
I am professional Tutor of of Business Courses, I did my four years Bachelor Degree from one of the Top Business schools of World "Institute of Business Administration" in year 2013. Since then I have been working as Tutor of Accounting, Finance tutor on different online platforms like this website. I am have experience of 6 years teaching business courses to students online and offline my professional job at national savings also helped me in accounting understanding .
4.90+
8+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
The following model is a representation of asparatame, C14H18N2O5, known commercially as NutraSweet. Only the connection between atoms is shown; multiple bonds are not indicated. Complete the...
-
The following model is specified: y1 = 1y2 + 11x1 + 1, y2 = 2y1 + 22x2 + 32x3 + 2. All variables are measured as deviations from their means. The sample of 25 observations produces the following...
-
The following model is specified:y 1 = 1 y 2 + 11 x 1 + 1 ,y 2 = 2 y 1 + 22 x 2 + 32 x 3 + 2 . All variables are measured as deviations from their means. The sample of 25 observations produces...
-
What are the premises for successful paleostress analysis?
-
Amber Company has used the dollar-value LIFO technique for the past three years. The company has only one inventory pool. Its beginning inventory for the current year was computed as follows: a. The...
-
In an article in IEEE Transactions on Instrumentation and Measurement (2001, Vol. 50, pp. 986990), researchers reported on a study of the effects of reducing current draw in a magnetic core by...
-
What strengths do they appear to bring to the organization? What weaknesses do they have?
-
The accountant for Ronaldo Company makes the assumptions or performs the activities listed below. Tell which of the following concepts of accrual accounting most directly relates to each assumption...
-
otomeducation.com Blackboard Learn Homework 3 - Chapters 6, 7, 88 Content SI 5.00 points At each calendar year-end, Mazie Supply Co. uses the percent of accounts receivable method to estimate bad...
-
written by Allen Schmidt, Julie E. Kendell, and Kenneth E. Kendall On a warm, sunny day in late October Chip Puller parks his car and walks into his office at Central Pacific University. It felt good...
-
The following model is representation of critic acid, the key substance in the so-called citric acid cycle by which food molecules is metabolized in the body. Only the connection between atoms is...
-
How many valence electrons does the each of the following dietary trace elements have? (a) Zinc (b) Iodine (c) Silicon (d) Iron
-
Using ca.Finance.Yahoo.com, download the monthly prices over a 5-year period for 5 Canadian companies of your choice. Calculate the monthly rates of return for each stock using "Adj. Close" (which...
-
Two roommates (Jen and Kate) can choose whether to clean their apartment (C) or leave it dirty (D). Jen's cost of cleaning is c, but Kate doesn't mind cleaning and has no cost. [Recall that their...
-
Designation Mass per Depth Width Thickness metre of of section section of of web flange Root Depth radius between Ratios for local buckling Second moment of area Radius of gyration fillets | i Flange...
-
Discuss the attributes that make an effective leader. What tenets should a leader practice? How does leadership directly impact effective public management? In your own experience, what has led you...
-
Given the following examples identify whether it describes a positive externality, negative externality, or neither. Example 1: Johanna is graduating from college this weekend. Like her, individuals...
-
32) Suppose Joaquin grows at an average rate of 0.5in/year for 3 years, then 1.25 inches/year for 4 years, then 0.75 inches/year for 4 years, then 0.4in/year for 5 years. In that time span, how much...
-
Discuss the behavioral implications of using actual profit contribution rather than budgeted profit contribution when calculating sales quantity and mix variances. LO.1
-
Fill in each blank so that the resulting statement is true. A solution to a system of linear equations in two variables is an ordered pair that__________ .
-
Prove that a. (), b. G; P,S,Nji - ( ). H - TS = G = (), aU N S,V,Nji V,T,Nji
-
Explain whether you would expect KBr or CH3Br to have the higher melting point.
-
Which of these isomers would you expect to have the higher boiling point: Explain? CH 3 CH 2 CH 2 OH or CH 3 CH 2 OCH 3
-
Which of these compounds would you expect to be more soluble in water? Explain? CHCHCHCHCOH or CH3CHCHCHCHCOH
-
question 6 Timely Inc. produces luxury bags. The budgeted sales and production for the next three months are as follows july. august september Sales, in units 1,115. 1229. 1302 Production. in units...
-
On May 12 Zimmer Corporation placed in service equipment (seven-year property) with a basis of $220,000. This was Zimmer's only asset acquired during the year. Calculate the maximum depreciation...
-
Power Manufacturing has equipment that it purchased 7 years ago for $2,550,000. The equipment was used for a project that was intended to last for 9 years and was being depreciated over the life of...

Study smarter with the SolutionInn App


