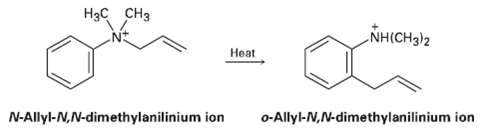
The following rearrangement of N-allyl-N, N-dimethyl anilinium ion has been observed. Propose amechanism. H H NH(CH3)2 eat
Question:
The following rearrangement of N-allyl-N, N-dimethyl anilinium ion has been observed. Propose amechanism.
Transcribed Image Text:
Hзс сHз NH(CH3)2 Нeat N-Allyl-N,N-dimethylanilinium ion o-Allyl-N,N-dimethylanilinium ion
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 53% (13 reviews)
H3C CH3 2N CH ...View the full answer

Answered By

ANDREW KIPRUTO
Academic Writing Expert
I have over 7 years of research and application experience. I am trained and licensed to provide expertise in IT information, computer sciences related topics and other units like chemistry, Business, law, biology, biochemistry, and genetics. I'm a network and IT admin with +8 years of experience in all kind of environments.
I can help you in the following areas:
Networking
- Ethernet, Wireless Airmax and 802.11, fiber networks on GPON/GEPON and WDM
- Protocols and IP Services: VLANs, LACP, ACLs, VPNs, OSPF, BGP, RADIUS, PPPoE, DNS, Proxies, SNMP
- Vendors: MikroTik, Ubiquiti, Cisco, Juniper, HP, Dell, DrayTek, SMC, Zyxel, Furukawa Electric, and many more
- Monitoring Systems: PRTG, Zabbix, Whatsup Gold, TheDude, RRDtoo
Always available for new projects! Contact me for any inquiries
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Dimethyl sulfoxide (DMSO) has been used as an anti-inflammatory rub for race horses. DMSO and acetone appear to have similar structures, but the C=O carbon atom in acetone is planar, while the S=O...
-
Propose a mechanism for the rearrangement that converts an into an (Section 22.6). a-hydroxyimine a-aminoketone Nhemoglobin CH2NH hemoglobin AU: OK as changed? rearrangement CH2OH CH,OH
-
The pKa of the anilinium ion (C6H5NH3) is 4.63. On the basis of this fact, decide whether aniline (C6H5NH2) is a stronger or weaker base than methylamine.
-
The unadjusted trial balance of Simple Consulting Services as at December 31, 2021 is as follows: Cash Accounts receivable Prepaid insurance Supplies inventory Office equipment Accumulated...
-
Fourth, executives need to directly participate in improvement projects not just "support" them .... By observing the successes and failures of improvement programs firsthand, rather than relying on...
-
What kind of hedging strategy should Kirk recommend for minimizing Pre-Fabs exposure to volatility in copper prices? Design a suitable hedge and show what would be the result if copper prices went to...
-
On your own, recapitulate the trinary classification analysis undertaken in this chapter using the Loans3 data sets. (Note that the results may differ slightly due to different settings in the CART...
-
Armstrong Chemical began operations in January. The company manufactures an acrylic car wax called Tough-Coat. The following standard cost estimates were developed several months before the company...
-
Which of the following is not required for CVP analysis a. Contribution margin b. Fixed expenses c. Variable costs d. Fixed expense per unit
-
During 2023, Emily worked as a financial controller for Vector Industries (VI) and earned a salary of $150,000. VI downsized its office space after the pandemic, and adopted a hybrid working policy...
-
Coronafacic acid, a bacterial toxin, was synthesized using a key step that involves three sequential pericyclic reactions. Identify them, and propose a mechanism for the overall transformation. I low...
-
Some people have suggested that understanding human behavior at work is the single most important requirement for managerial success. Do you agree or disagree with this statement? Why?
-
How have media ownership restrictions changed since the Telecommunications Act of 1996 was passed and what impact has that had on the media industry?
-
2. Question 2 When preparing a financial spread analysis, what should be done when the financial statement captions don't align with those provided in the spread template? 1 point Conform the...
-
Your company just secured an $6 million contract with a major public-sector client that is expected to generate thousands of jobs over the next 10 years. Describe the scenario as a blog.
-
Dr. John Gottman's research has been able to accurately predict divorce more than 90% of the time.By carefully studying how couples interact with each other, he identified what are known as "The Four...
-
Adult Sleep Times (hours) of sleep for randomly selected adult subjects included in the National Health and Nutrition Examination Study are listed below. Here are the statistics for this sample: n =...
-
For high-energy electron diffraction in a TEM, another estimate of the precision of diffraction angles can be provided by the uncertainty principle: px We do not know the specific plane that scatters...
-
Do you typically ask a lot of questions? What can others do to either encourage or inhibit you from feeling comfortable in this manner?
-
Determine the reactions in supports A and D and connections B and C. Sketch its shear and moment diagram and determine the magnitude ankoration of the maximum shear and moment for every member. 18 3...
-
When the NYSE reduced the typical minimum tick size (also known as a Minimum Price VariantMPV) from 1/8 to 0.01, spreads and transactions costs were reduced, resulting in, according to most...
-
(a) What compound of molecular formula C6H10 gives 2,3-dimethylbutane on catalytic hydrogenation over platinum? (b) What two compounds of molecular formula C11H20 give 2,2,6,6-tetramethylheptane on...
-
From among the following compounds, choose the two that yield the same carbocation on ionization. CH3 CH3 CH3 C CHa CH3 Cl Br
-
Write structural formulas for all the (a) Conjugated dienes (b) Isolated dienes (c) Cumulated dienes that give 2,4-dimethylpentane on catalytic hydrogenation.
-
Practice Problem 1 The stockholders equity accounts of Bramble Corp. on January 1, 2017, were as follows. Preferred Stock (6%, $100 par noncumulative, 4,400 shares authorized) $264,000 Common Stock...
-
JVCU Which of the following is considered cash for financial reporting purposes? 1 JVCU Which of the following is considered cash for financial reporting purposes? 1
-
Required information The Foundational 15 [LO8-2, LO8-3, LO8-4, LO8-5, LO8-7, LO8-9, L08-10) (The following information applies to the questions displayed below.) Morganton Company makes one product...

Study smarter with the SolutionInn App


