Question: The Kelvin equation, (17-16), predicts that solubility increases to infinity as the crystal diameter decreases to zero. However, measurements by L. Harbury [J. Phys. Chem.,
The Kelvin equation, (17-16), predicts that solubility increases to infinity as the crystal diameter decreases to zero. However, measurements by L. Harbury [J. Phys. Chem., 50, 190-199 (1946)] for several inorganic salts in water show a maximum in the solubility curve and a solubility that approaches zero as crystal size is reduced to zero. Harbury's explanation is that the surface energy of the crystals depends not only on interfacial tension, but also on surface electrical charge, given by
![]()
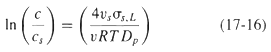
where
q = electrical charge on the crystal
K = dielectric constant
Modify (17-16) to take into account electrical charge. Make sure your equation predicts amaximum.
2q v;/TKRT D
Step by Step Solution
3.43 Rating (166 Votes )
There are 3 Steps involved in it
From 1716 To predict a maximum modify 1 by in... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
37-E-C-E-S-P (582).docx
120 KBs Word File


