The liquid-liquid extractor in figure operates at 100F and a nominal pressure of 15 psia. For the
Question:
The liquid-liquid extractor in figure operates at 100°F and a nominal pressure of 15 psia. For the feed and solvent flows shown, determine the number of equilibrium stages to extract 99.5% of the acetic acid, using the NRTL equation for activity coefficients. The NRTL constants may be taken as follows:
1 = ethyl acetate
2 = water
3 = acetic acid
Compare the computed compositions of the raffinate and extract products to those of figure.
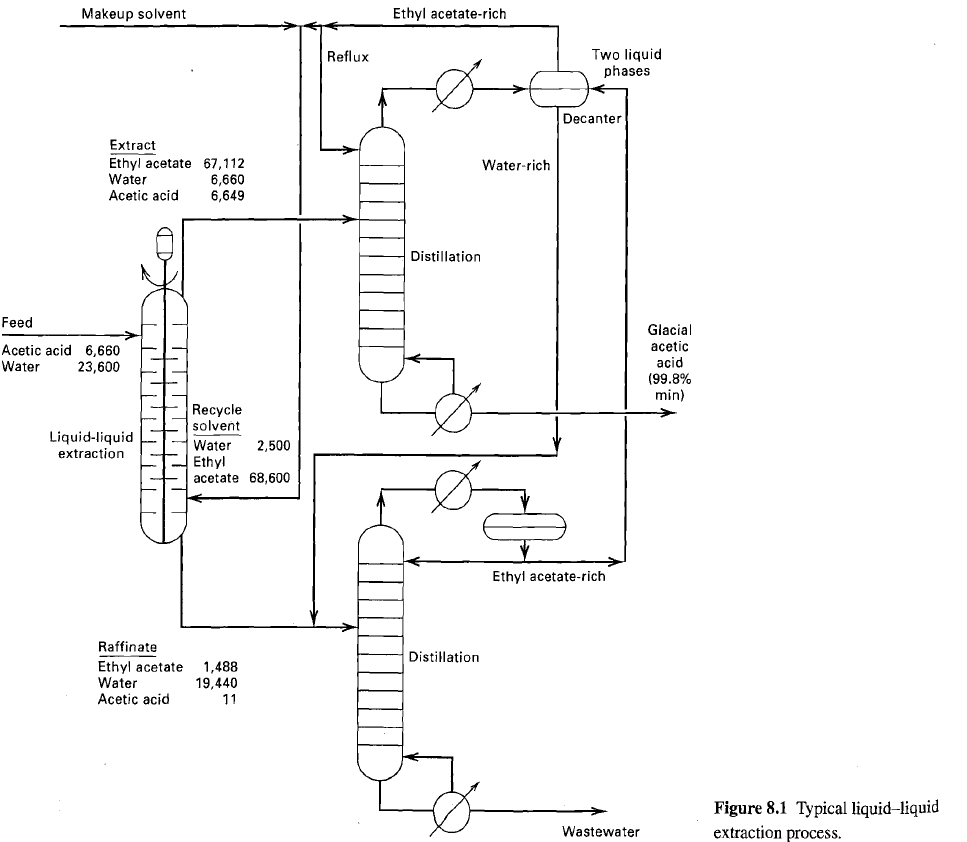
Transcribed Image Text:
Ethyl acetate-rich Makeup solvent Two liquid phases Reflux Decanter Extract Ethyl acetate 67,112 Water Acetic acid Water-rich 6,660 6,649 Distillation Feed Glacial acetic acid Acetic acid 6,660 23,600 Water (99.8% min) Recycle solvent Liquid-liquid extraction Water Ethyl acetate 68,600 2,500 Ethyl acetate-rich Raffinate Distillation Ethyl acetate Water Acetic acid 1,488 19,440 11 Figure 8.1 Typical liquid–liquid extraction process. Wastewater
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (18 reviews)
The Extract model of Chemcad was used starting with 2 equilibrium stages an...View the full answer

Answered By

Pushpinder Singh
Currently, I am PhD scholar with Indian Statistical problem, working in applied statistics and real life data problems. I have done several projects in Statistics especially Time Series data analysis, Regression Techniques.
I am Master in Statistics from Indian Institute of Technology, Kanpur.
I have been teaching students for various University entrance exams and passing grades in Graduation and Post-Graduation.I have expertise in solving problems in Statistics for more than 2 years now.I am a subject expert in Statistics with Assignmentpedia.com.
4.40+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Chemical Engineering questions
-
The network in figure operates at f = 400 Hz. Use PSPICE to find the current Io. 1, :-j2n 12/0 v (+ 10 Ao0/9 (+
-
The network in figure operates at f = 60Hz. Use PSPICE to find the voltage vo. 320 10 10 2/0 A 12/0Pv 10 V j2n
-
The total pressure of vapor over liquid acetic acid at 71.3°C is 146 mmHg. If the density of the vapor is 0.702 g/L, what is the mole fraction of dimer in the vapor? See Problem 11.149. Problem...
-
Claud Chapperon is a self-employed distributor of wholesale clothing who began trading on 1 July 2012. His summarised accounts for the year to 30 June 2020 are shown below. The figures in brackets...
-
Andre is puzzled reading Transat A.T. (Air Transat) Inc.'s financial statements. He notices that the numbers have all been rounded to the nearest thousand. He thought financial statements were...
-
List 10 items that must be specified for pumps.
-
A consumer group would like to compare the satisfaction ratings of three fast-food restaurants: McDoogles, Burger Queen, and Windys. A random sample of satisfaction scores on a scale of 120 was...
-
The following data were taken from the balance sheet accounts of Symbol Two Corporation on June 30, 2014. Current assets ............ $125,000 Investments ............ 365,000 Common stock (par value...
-
Statement of Cash Flows The comparative balance sheet of Whitman Co. at December 31, 20Y2 and 2041, is as follows: Dec. 31, Dec. 31, 2012 2011 Assets Cash $ 823,990 $ 887,760 683,910 749,830...
-
You are employed by McDowell and Partners, Chartered Accountants (M&P). A new client, Community Finance Corporation (CFC), approached M&P for assistance. Enviro Ltd. (Enviro) has asked CFC for a loan...
-
A mixture of cyclohexane and cyclopentane is to be separated by liquid-liquid extraction at 25 o C with methanol. Phase equilibria for this system may be predicted by the NRTL or UNIQUAC equations....
-
For the ternary system, normal hexane-methanol-methyl acetate at 1 atm find, in suitable references, all the binary and ternary azeotropes, sketch an approximate residue-curve map on a...
-
Choose the correct verb form. The data on my computer (was/were) lost when the hard drive failed.
-
Question (4) seen, 20 vehicles/km moving at 100 km/h and 30 vehicles/km traveling at 120 km/h. Two successive videos showing stationary traffic on the road were examined. Two groups of platoons were...
-
?In civil engineering, what is the main use of a slump test in concrete technology?
-
Explain the process of compression resin transfer molding(CRTM)?in composite manufacturing. What are the benefits of using CRTM for producing composite structures?
-
Explore the role of post-occupancy evaluation in commercial and industrial architecture. How do architects use feedback from building users to improve future designs?
-
Discuss the principles of geotechnical engineering in slope stability analysis. How can engineers assess slope stability, mitigate landslide risks, and design effective stabilization measures to...
-
Real Options How would the analysis of real options change if a company has competitors?
-
In muscle tissue, the ratio of phosphorylase a to phosphorylase b determines the rate of conversion of glycogen to glucose 1phosphate. Classify how each event affects the rate of glycogen breakdown...
-
Would a wealthy individual with bank accounts of more than $100,000 prefer the FDIC to use the purchase and assumption method or the payoff method to liquidate failed banks? Why?
-
The separation of propane and propylene is accomplished by distillation, but at the expense of more than 100 trays and a reflux ratio greater than 10. Consequently, the use of adsorption has been...
-
Shen and Smith [Ind. Eng. Chem. Fundam., 7, 100105 (1968)] measured equilibrium-adsorption isotherms at four temperatures for benzene vapor on silica gel having the following properties: S g = 832 m...
-
The following data were obtained in a BET apparatus for adsorption equilibrium of N 2 on silica gel (SG) at 195.8C. Estimate Sg in m 2 /g of silica gel. How does your value compare with that in Table...
-
Compute the value of ordinary bonds under the following circumstances assuming that the coupon rate is 0.06:(either the correct formula(s) or the correct key strokes must be shown here to receive...
-
A tax-exempt municipal bond has a yield to maturity of 3.92%. An investor, who has a marginal tax rate of 40.00%, would prefer and an otherwise identical taxable corporate bond if it had a yield to...
-
Please note, kindly no handwriting. Q. Suppose a 3 year bond with a 6% coupon rate that was purchased for $760 and had a promised yield of 8%. Suppose that interest rates increased and the price of...

Study smarter with the SolutionInn App


