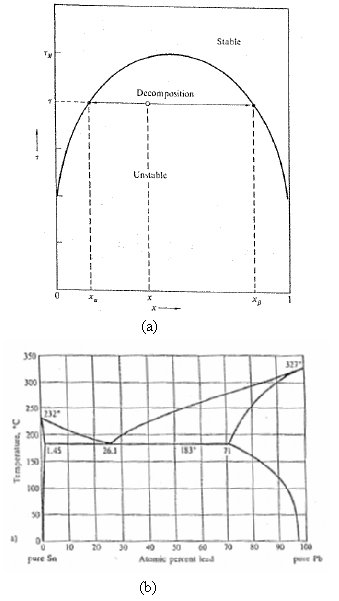
The phase diagram of liquid 3 He 4 He mixtures in Figure shows that the solubility of
Question:
The phase diagram of liquid 3He– 4He mixtures in Figure shows that the solubility of 3He in 4He remains finite (about 6 pct) as τ → 0. Similarly, the Pb-Sn phase diagram of Figure shows a finite residual solubility of Pb in solid Sn with decreasing τ. What do such finite residual solubilities imply about the form of the function u(x)?
Transcribed Image Text:
Stabte Decomposition Unstable (a) 350 327 300 250 232 200 L.4S 190 I83 26.1 71 00 a) 20 80 10 20 30 30 90 100 pure Se Atomis peneni lead (b) Temperature, "C
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (12 reviews)
The curve ux must have a shape qualitatively ...View the full answer

Answered By

Lilian Nyambura
Hi, am Lilian Nyambura, With extensive experience in the writing industry, I am the best fit for your writing projects. I am currently pursuing a B.A. in Business Administration. With over 5 years of experience, I can comfortably say I am good in article writing, editing and proofreading, academic writing, resumes and cover letters. I have good command over English grammar, English Basic Skills, English Spelling, English Vocabulary, U.S. English Sentence Structure, U.K. or U.S. English Punctuation and other grammar related topics. Let me help you with all your essays, assignments, projects, dissertations, online exams and other related tasks. Quality is my goal.
4.80+
378+ Reviews
750+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Solid State questions
-
The phase diagram of sulfur is shown here. (a) How many triple points are there? (b) Monoclinic and rhombic are two allotropes of sulfur. Which is more stable under atmospheric conditions? (c)...
-
The phase diagram of a hypothetical substance is (a) Estimate the normal boiling point and freezing point of the substance. (b) What is the physical state of the substance under the following...
-
Use graph paper and sketch the phase diagram of oxygen, O2, from the following information: normal melting point, 218C; normal boiling point, 183C; triple point, 219C, 1.10 mmHg; critical point,...
-
Southern Stitches is a local casual clothing shop that makes a variety of t-shirt styles. Below is some actual vs. budget information for Southern's t-shirts for the month of May. Assume no...
-
This problem illustrates a deceptive way of quoting interest rates called add-on interest. Imagine that you see an advertisement for Crazy Judy's Stereo City that reads something like this: "$1,000...
-
Refer to Exercise 91. Here are a boxplot and some numerical summaries of the electoral vote data: a. Explain why the median and IQR would be a better choice for summarizing the center and variability...
-
What are the four common methods for binning numerical predictors? Which of these are preferred? Use the following data set for Exercises 2830: 111337
-
Recognizing cash flows for capital investment projects, NPV. Met-All Manufacturing manufactures over 20,000 different products made from metal, including building materials, tools, and furniture...
-
Gloria Black was new employee to ABC Business, she is a single parent living with her 17-year-old son---Mark, who studied fulltime (four months) this year. Mark's tuition fees for 2018 are $2000....
-
Required: Based upon what you find, answer the following questions: A. What is the return on assets for Big Store (a) in 2012 and (b) in 2021? (Round your answers to 2 decimal places.) B. What is the...
-
Show that the chemical potentials A and B of the two atomic species A and B of an equilibrium two phase mixture are given by the intercepts of the two-point tangent in Figure with the vertical...
-
Let B be an impurity in A, with X < < 1. In this limit the non-mixing parts of the free energy can be expressed as linear functions of x, as f0(x) = f0(0) + xf0(0), for both liquid and solid phase....
-
Changing the order in a sequence of transformations may change the final result. Investigate each pair of transformations in Problem to determine if reversing their order can produce a different...
-
Analysis of the Volkswagen Scandal Possible Solutions for Recovery The Volkswagen scandal is a notorious example of how corporations can shape the ethical and political issues of the environment. The...
-
Shelby isn't sure if her forklift can safely handle the pallet she is being asked to move. What can she check to be sure
-
If schedule acceleration increases costs, how could schedule elongation reduce costs? If schedule acceleration increases costs, how could schedule elongation reduce costs? For the same total...
-
Laser Care Hospital is looking to raise tax-exempt municipal funds in the bond market. As an issuer of the bond, which of the following is not a part of the bond process that Laser Care Hospital will...
-
Find the critical value t a/2 corresponding to a 95% confidence level. (13.046, 22.15) X= 17.598 Sx= 16.01712719 n=50
-
P15-7B Research indicates that consumers prefer upscale restaurants. To capitalize on this trend, Palomino Eurobistros. Inc., is embarking on a massive expansion. Plans call for open- ing 20 new...
-
In the current year, the City of Omaha donates land worth $500,000 to Ace Corporation to induce it to locate in Omaha and create an estimated 2,000 jobs for its citizens. a. How much income, if any,...
-
Write the SQL code for the following: Order the reservations by class date and then by class ID. Display all fields. a. SELECT * FROM Reservations ORDER BY ClassDate, ClassID; b. DISPLAY ALL FROM...
-
One mole of an ideal gas is contained in a cylinder with a movable piston. The initial pressure, volume, and temperature are Pi, Vi, and Ti, respectively. Find the work done on the gas for the...
-
One mole of an ideal gas, initially at 300 K, is cooled at constant volume so that the final pressure is one fourth of the initial pressure. Then the gas expands at constant pressure until it reaches...
-
Continue the analysis of Problem 60 in Chapter 19. Following a collision between a large spacecraft and an asteroid, a copper disk of radius 28.0 m and thickness 1.20 m, at a temperature of 850C, is...
-
Deacon Company is a merchandising company that is preparing a budget for the three - month period ended June 3 0 th . The following information is available Deacon Company Balance Sheet March 3 1...
-
Mango Company applies overhead based on direct labor costs. For the current year, Mango Company estimated total overhead costs to be $460,000, and direct labor costs to be $230,000. Actual overhead...
-
Which of the following do we expect to be the horizon growth rate for a company (long term growth rate- say 30-50 years)? A) Inflation B) Industry Average C) Zero D) Market Beta

Study smarter with the SolutionInn App


