The relative volatility, a, of benzene to toluene at 1 atm is 2.5. Construct an x-y diagram
Question:
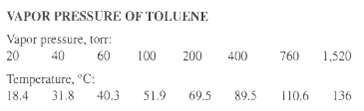
The relative volatility, a, of benzene to toluene at 1 atm is 2.5. Construct an x-y diagram for this system at 1 atm. Repeat the construction using vapor pressure data for benzene from Exercise 4.6 and for toluene from the following table in conjunction with Raoult's and Dalton's laws. Also construct a T-x-y diagram.
(a) A liquid containing 70 mol% benzene and 30 mol% toluene is heated in a container at 1 atm until 25 mol% of the original liquid is evaporated. Determine the temperature. The phases are then separated mechanically, and the vapors condensed. Determine the composition of the condensed vapor and the liquid residue.
(b) Calculate and plot the K-values as a function of temperature at 1atm.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





