The vapor pressure of toluene is given in Exercise 4.8, and that of n-heptane is given in
Question:
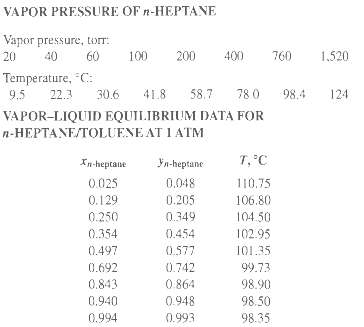
The vapor pressure of toluene is given in Exercise 4.8, and that of n-heptane is given in the accompanying table.
(a) Plot an x-y equilibrium diagram for this system at 1 atm by using Raoult's and Dalton's laws.
(b) Plot the T-x bubble-point curve at 1 atm.
(c) Plot a and K-values versus temperature.
(d) Repeat part (a) using the arithmetic average value of a, calculated from the two extreme values.
(e) Compare your x-y and T-x-y diagrams with the following experimental data of Steinhauser and White.
Transcribed Image Text:
VAPOR PRESSURE OF n-HEPTANE Vapor pressure, torr: 60 100 200 400 760 1,520 20 40 Temperature, "C: 58.7 41.8 78 0 98.4 9.5 22.3 30.6 124 VAPOR-LIQUID EQUILIBRIUM DATA FOR n-HEPTANE/TOLUENE AT I ATM T,°C Xn-heptane Fn-heptane 0.048 0.025 110.75 0.129 0.205 106.80 0.250 0.349 104.50 0.354 0.454 102.95 0.497 0.577 101.35 0.692 0.742 99.73 0 864 0.843 98.90 0.940 0.948 98.50 0.994 0.993 98.35
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 53% (15 reviews)
a To calculate yx and Txy curves from vapor pressure data using Raoults and Daltons laws Eq 244 appl...View the full answer

Answered By

Caroline Kinuthia
Taking care of the smaller details in life has a larger impact in our general well being, and that is what i believe in. My name is Carol. Writing is my passion. To me, doing a task is one thing, and delivering results from the task is another thing. I am a perfectionist who always take things seriously and deliver to the best of my knowledge.
4.90+
1934+ Reviews
4277+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Chemical Engineering questions
-
The following table gives the vapor pressure of hexafluorobenzene (C6F6) as a function of temperature: (a) By plotting these data in a suitable fashion, determine whether the Clausius-Clapeyron...
-
In Section 11.5 we defined the vapor pressure of a liquid in terms of an equilibrium. (a) Write the equation representing the equilibrium between liquid water and water vapor and the corresponding...
-
The vapor pressure of a volatile liquid can be determined by slowly bubbling a known volume of gas through the liquid at a given temperature and pressure. In an experiment, a 5.40-L sample of...
-
Once an LLC has engaged in a dissolution procedure, the business does not stop immediately. True/False
-
The financial statements of Anglo American Platinum Limited (Amplats) are presented in Appendix B following the financial statements for Eastern Platinum Limited (Eastplats) in Appendix A....
-
On January 1, 2018, a machine was installed at Dunbar Factory at a cost of $53,000. Its estimated residual value at the end of its estimated life of 4 years is $20,000. The machine is expected to...
-
Does race affect use of the Internet in various ways? Using the PewWorkPlay dataset, run two regressions using the two Internet use indexes as your dependent variables and the two race reference...
-
At a recent meeting of the accounting staff in your company, the controller raised the issue of using present value techniques to conduct impairment tests for some of the companys fixed assets. Some...
-
The Cutting Department of Cassel Company has the following production and cost data for July. Production Costs 1. Transferred out 14,000 units. Beginning work in process $0 2. Started 3,100 units...
-
Reconsider the example of choosing the advertising budget at VRX that is presented in Section 1.4. In addition to the VRX2000, VRX also has a higher-end virtual-reality headset, the VRX3000. This...
-
The relative volatility, a, of benzene to toluene at 1 atm is 2.5. Construct an x-y diagram for this system at 1 atm. Repeat the construction using vapor pressure data for benzene from Exercise 4.6...
-
Saturated-liquid feed, of F = 40 mollh, containing 50 mol% A and B is supplied continuously to the apparatus shown in Figure. The condensate from the condenser is split so that half of it is returned...
-
Lewis owned a vacant building. He persuaded Dinkelspeel to open and to conduct a business called The Buffet in the property. Together, they purchased furniture, fixtures, and merchandise. They agreed...
-
What is brand awareness for Jam & Daisies ? their leaning advantage, consideration advantage, choice advantages? 5. what is the recommendation of brand awareness? 6. What is Brand recognition? 7....
-
On August 1st, Custom Car Co's work in process inventory was $24900; its raw materials inventory was $6000; manufacturing overhead had a $1800 debit balance. Work in Process Subsidiary Data 8/1:...
-
Case: Castoro & Partners, CPAs is auditing Cloud 9 for the FY2023. Cloud 9 is a small public company and has been an audit client of Castoro & Partners since 2018. Materiality Methodology: Overall...
-
1)Solve the following differential equations by Undetermined Coefficient Method. dy dx dy - 4- 4+ 4y = 16x2e2x dx
-
Every year Monty Industries manufactures 8,600 units of part 231 for use in its production cycle. The per unit costs of part 231 are as follows: Direct materials Direct labor Variable manufacturing...
-
Liquidated Damages versus Penalties. Every homeowner in the Putnam County, Indiana, subdivision of Stardust Hills must be a member of the Stardust Hills Owners Association, Inc., and must pay annual...
-
A non-charmed baryon has strangeness S = 2 and electric charge Q = 0. What are the possible values of its isospin I and of its third component I z ? What is it usually called if I = 1/2?
-
Explain whether an equity fund is likely to use performance figures in a print advertisement if marketing an equity fund with a high relative 1-year total return, but with low or negative 5- and...
-
What is the difference between a Class 1 and a Class 2 separation? Why is the Class 1 Underwood equation useful even if the separation is Class 2?
-
What is a pinch point or region? For multicomponent distillation, under what conditions is the pinch point located at the feed location? What conditions cause the pinch point to migrate away from the...
-
For the conditions of Exercise 9.7, with bubble-point liquid feed at column pressure, compute the minimum external reflux and nonkey distribution at Rmin by the Class 2 Underwood equations. Data From...
-
Question 24 Not yet answered Marked out of 1.00 P Flag question Muscat LLC's current assets and current liabilities are OMR 258,000 and OMR 192,000, respectively. In the year 2020, the company earned...
-
Question 24 Miami Company sold merchandise for which it received $710,400, including sales and excise taxes. All of the firms sales are subject to a 6% sales tax but only 50% of sales are subject to...
-
f the IRS intends to close a Taxpayer Assistance Center, they must notify the public at least _____ days in advance of the closure date. 14 30 60 90

Study smarter with the SolutionInn App


