The scattering of electrons or neutrons from a pair of nuclei separated by a distance Rij and
Question:
The scattering of electrons or neutrons from a pair of nuclei separated by a distance Rij and orientated at a definite angle to the incident beam can be calculated. When the molecule consists of a number of atoms, we sum over the contribution from all pairs, and find that the total intensity has an angular variation given by the Wierl equation:
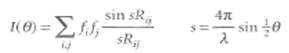
Where cis the wavelength of the electrons in the beam and 8 is the scattering angle the electron scattering factor.], is a measure of the intensity of the electron scattering powers of the atoms.
(a) Predict from the Wierl equation the positions of the first maximum and first minimum in the neutron and electron diffraction patterns of a Br, molecule obtained with neutrons of wavelength 78 pm wavelength and electrons of wavelength 4.0 pm.
(b) Use the Wierl equation to predict the appearance of the 10.0 keV electron diffraction pattern ofCCI4 with an (as yet) undetermined C-Cl bond length but of known tetrahedral symmetry. Take fCI = 17f and fC = 6f and note that R (CI, CI) = (8/3)1/2R(C, CI). Plot lit' against positions of the maxima, which occurred at 3° 0', 5° 22', and 7° 54', and minima, which occurred at 1° 46', 4° 6', 6° 40', and 9° 10' what is the C-CI bond length in CCI/
Step by Step Answer:






