The specific rotation of the R enantiomer of the following alkene is a20D = +76 degree mL
Question:
The specific rotation of the R enantiomer of the following alkene is a20D = +76 degree mL g-1 dm-1, and its molecular mass is 146.2.
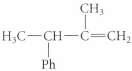
Transcribed Image Text:
CH HC CHC CH Ph
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 53% (15 reviews)
The rotation of the enantiomer cancels h...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
(a) Draw line-and-wedge structures for the two enantiomers of the following allene. (b) One enantiomer of this compound has a specific rotation of - 30.7o. What is the specific rotation of the other?...
-
What observed rotation is expected when a 1.5 M solution of (R)-2-butanol is mixed with an equal volume of a 0.75 M solution of racemic 2-butanol, and the resulting solution is analyzed in a sample...
-
(a) The specific rotation of sucrose (table sugar) in water is 66.5 degrees mL g-1 dm-1. What is the observed optical rotation in a 1 dm path of a sucrose solution prepared from 5 g of sucrose and...
-
On January 1, 2019, Chiz Company acquired equipment to be used in its manufacturing operations. The equipment has an estimated useful life of 10 years and an estimated residual value of P50,000. The...
-
What are the probable effects on sales and profits of each of the following credit these policies?
-
The concept of merchandising.
-
P 5-6 Upstream and downstream sales, 90 percent owned Justin Bhd is a 90 percent-owned company of Epik Bhd and was acquired in 2011, when the book value of Justin Bhds net identifiable assets were...
-
Now that you have read the chapter on financial planning, what do you recommend to Austin for his talk with Rachel on the subject of financial planning regarding? 1. Setting financial goals? 2....
-
Profit center responsibility reporting for a service company Instructions Quarterly Income Statements Final Questions Instructions Red Line Railroad Inc has three regional divisions organized as...
-
Blue Computers, a major server manufacturer in the United States, currently has plants in Kentucky and Pennsylvania. The Kentucky plant has a capacity of 1 million units a year, and the Pennsylvania...
-
(a) Draw sawhorse projections of ephedrine (Problem 6.32) about the C1-C2 bond for all three staggered and all three eclipsed conformations. (b) Examine each conformation for chirality. How do the...
-
What is the absolute configuration of (+) -methyl- hexane if catalytic hydrogenation of (S)-(+)-3- methyl-1-hexene gives (-)-3-methylhexane?
-
You have a random sample of 10 population members from each of two normal populations, and you want to set up a test using the following hypotheses: H 0 : 1 2 = 0 H a : 1 2 0 a. Using a...
-
Explain the importance(s) of the Teamwork soft skill in health care. Describe in detail an example of how it may be used in a healthcare setting.
-
Write a solution to this problem in the main method of a class named " Money " Ask the user to enter a number representing an amount of money from 1 dollar to 9999 dollars (integer). Assume the user...
-
Use linspace to define -4
-
Although beer may be the beverage of choice for most 20 somethings, for 28-year-old Geoff Dillon, his drink of choice would likely be whisky. Dillon grew up watching his dad, an environmental chemist...
-
In our text, the author discusses five drivers of a green supply chain. While each is important, different companies may be more influenced by some more than others. In your discussion, give an...
-
Do principal and interest remain constant over time? Explain your answer.
-
Express these numbers in standard notation. a. 2.87 10-8 b. 1.78 1011 c. 1.381 10-23
-
Draw a conventional structure corresponding to the following skeletal structure, and then name it.
-
Which of the following alkenes can exist as double-bond stereoisomers? Identify the stereocenters in each. (a) H,C=CHCH,CH,CH, (b) CH,CH,CH=CHCH,CH, 1-pentene 3-hexene (c) HC=CH-CH=CH-CH3 (d)...
-
Name the following compound using IUPAC substitutive nomenclature. HC=CCH,CH,CH, I CH,CH,CH,CH,CH,
-
THIS IS ONE QUESTION WITH TWO PARTS. PLEASE ANSWER COMPLETELY AND SHOW ALL WORK. (NO EXCEL) Information for Question 1: State Probability Retum on A Return on B Return on C Retum on Portfolio X Boom...
-
Direct materials (5.0 Ibs. @ $5.00 per Ib.) Direct labor (2.0 hrs. @ $13.00 per hr.) Overhead (2.0 hrs. @ $18.50 per hr.) Total standard cost $25.00 26.00 37.00 $88.00 The predetermined overhead rate...
-
Problem 1-28 (Algo) (LO 1-4, 1-5, 1-6b 1-7) Harper, Inc., acquires 40 percent of the outstanding voting stock of Kinman Company on January 1, 2020, for $316,100 in cash. The book value of Kinman's...

Study smarter with the SolutionInn App


