There are five resonance structures of Phenanthrene, one of which is shown. Draw the otherfour. Phenanthrene
Question:
There are five resonance structures of Phenanthrene, one of which is shown. Draw the otherfour.
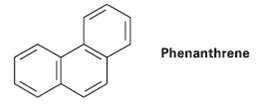
Transcribed Image Text:
Phenanthrene
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 76% (13 reviews)
The circled bond is represented as a doubl...View the full answer

Answered By

Fahmin Arakkal
Tutoring and Contributing expert question and answers to teachers and students.
Primarily oversees the Heat and Mass Transfer contents presented on websites and blogs.
Responsible for Creating, Editing, Updating all contents related Chemical Engineering in
latex language
4.40+
8+ Reviews
22+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
There are two contributing resonance structures for an anion called acetaldehyde enolate, whose condensed molecular formula is CH2CHO-. Draw the two resonance contributors and the resonance hybrid,...
-
Draw contributing resonance structures and a hybrid resonance structure that explain two related facts: the carbon-oxygen bond distances in the acetate ion are the same, and the oxygen's of the...
-
Draw two resonance structures for diazomethane, CH2N2. Show formal charges. The skeletal structure of the molecule is C N N
-
Based on your reading of the rules of Professional Code of Conduct as given by AICPA, which part of the code did the accountants of Enron, Waste Management or Wells Fargo have violated and how about...
-
What pros and cons of downsizing do you think apply to this example?
-
Lee Development Co. has found a site that it believes will support 75 homesites. The company also believes that the land can be purchased for $225,000 while direct development costs will run an...
-
Discuss why aligning the development of an SMIS with the organizations strategy is important. Describe the process of developing an effective SMIS. List four common social media goals and describe...
-
In Problem 11-27, a linear program was developed for the shortest-route problem. Modify this linear program to make the changes detailed in Problem 11-26. Solve this problem and compare it to the...
-
please read the article "HOW DO CFOS MAKE CAPITAL BUDGETINGAND CAPITAL STRUCTURE DECISIONS?" by John Graham and Campbell Harvey, Duke University what are the most important points in this article?...
-
The following balance sheet was prepared for Atlas Services Co. a. List any errors in the preceding balance sheet. b. Prepare a corrected balance sheet. ATLAS SERVICES cO. Balance Sheet For the Year...
-
There are four resonance structures for anthracene, one of which is shown. Draw the otherthree. Anthracene
-
Look at the five resonance structures for Phenanthrene (Problem 15.26) and predict which of its carboncarbon bonds is shortest. Phenanthrene
-
Write a GUI program that displays a 24 tree.
-
Let $N$ be a positive integer. Consider the relation $\circledast$ among pairs of integers $r, s \in \mathbb{Z}$ defined as $r \circledast s$ when $r-s$ is an integer multiple of $N$. Prove that...
-
Draw a circuit diagram for a typical home hair dryer. To which form (or forms) of energy is electric potential energy converted when you use the dryer?
-
Draw a vector field diagram for particles carrying charges \(+2 q\) and \(-q\) separated by a distance \(r\). Comment on the significance of the vector diagram.
-
(a) Show that the Jones matrix of a polarization analyzer set at angle \(\alpha\) to the \(X\)-axis is given by \[ \underline{\mathbf{L}}(\alpha)=\left[\begin{array}{cc} \cos ^{2} \alpha & \sin...
-
Let \(\mathbf{V}(t)\) be a linearly filtered complex-valued, wide-sense stationary random process with sample functions given by \[ \mathbf{v}(t)=\int_{-\infty}^{\infty} \mathbf{h}(t-\tau)...
-
describe the nature of a group of companies and explain the reasons for using a group structure;
-
What is removed during each of the three stages of wastewater treatment: primary, secondary, and tertiary? During which state would you expect items to be recovered that were accidentally flushed,...
-
The reaction shown here has a K p = 4.5 * 10 2 at 825 K. Find K c for the reaction at this temperature. CH(g) + CO(g) 2 CO(g) + 2 H(g)
-
The Sanger method for N-terminus determination is a less common alternative to the Edman degradation. In the Sanger method, the peptide is treated with the Sanger reagent, 2, 4-dinitrofluorobenzene,...
-
Show where trypsin and chymotrypsin would cleave the following peptide. Tyr-Ile-Gln-Arg-Leu-Gly-Phe-Lys-Asn-Trp-Phe-Gly-Ala-Lys-Gly-Gln-Gln NH2
-
After treatment with peroxyformic acid, the peptide hormone vasopressin is partially hydrolyzed. The following fragments are recovered. Propose a structure for vasopressin. Phe-Gln-Asn...
-
The Regal Cycle Company manufactures three types of bicyclesa dirt bike, a mountain bike, and a racing bike. Data on sales and expenses for the past quarter follow: Total Dirt Bikes Mountain Bikes...
-
?? A local college is deciding whether to conduct a campus beautification initiative that would imvolve various projects, such as planting trees and remodeling bulidings, to make the campus more...
-
A company has net income of $196,000, a profit margin of 9.7 percent, and an accounts receivable balance of $135,370. Assuming 70 percent of sales are on credit, what is the companys days sales in...

Study smarter with the SolutionInn App


