There are four resonance structures for anthracene, one of which is shown. Draw the otherthree. Anthracene
Question:
There are four resonance structures for anthracene, one of which is shown. Draw the otherthree.
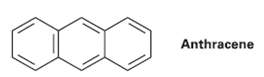
Transcribed Image Text:
Anthracene
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 42% (7 reviews)

Answered By

Jonas Araujo
I have recently received the degree of PhD. In Physics by the Universidade Federal do Maranhão after spending a term in Durham University, as I have been awarded a scholarship from a Brazilian mobility program. During my PhD. I have performed research mainly in Theoretical Physics and published works in distinguished Journals (check my ORCID: https://orcid.org/0000-0002-4324-1184).
During my BSc. I have been awarded a scholarship to study for a year in the University of Evansville, where I have worked in detection-analysis of photon correlations in the the Photonics Laboratory. There I was a tutor in Electromagnetism, Classical Mechanics and Calculus for most of that year (2012).
I am very dedicated, honest and a fast learner, but most of all, I value a job well done.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
For your analysis, choose 10 countries, one of which is the United States. Create a table showing whether each country applies a worldwide or territorial approach to international income taxation....
-
For your analysis, choose 10 countries, one of which is the United States. Create a table showing whether each country applies a worldwide or territorial approach to international income taxation....
-
There are two contributing resonance structures for an anion called acetaldehyde enolate, whose condensed molecular formula is CH2CHO-. Draw the two resonance contributors and the resonance hybrid,...
-
Consider the following reaction at 800. K: N2(g) + 3F2(g) 2NF3(g) An equilibrium mixture contains the following partial pressures: PN2 = 0.021 atm, PF2 = 0.063 atm, and PNF3 = 0.48 atm. Calculate Go...
-
Recruiting people for jobs that require international assignments is increasingly important for many organizations. Where might an organization go to recruit people interested in such assignments?
-
Treetop Associated Group (TAG) is seeking financing for acquisition and development of 147 homesites. The land will cost $1.5 million, and TAG estimates direct development costs to be an additional...
-
Define enterprise social network (ESN), and describe the primary goal of an ESN. Define Web 2.0 and Enterprise 2.0. Explain each element of the SLATES model. Explain how changes in communication...
-
The management of Easterling Corp. is considering the effects of various inventory-costing methods on its financial statements and its income tax expense. Assuming that the price the company pays for...
-
In Oman, government knows what is the best and most beneficial for the individuals and the entire economy. All the products are produced with high quality with reasonable prices so that majority of...
-
A counter flow, twin-tube heat exchanger is made by brazing two circular nickel tubes, each 40 m long, together as shown below. Hot water flows through the smaller tube of 10-mm diameter and air at...
-
Look at the three resonance structures of naphthalene shown in Section 15.7, and account for the fact that not all carboncarbon bonds have the same length. The C1C2 bond is 136 pm long, whereas the...
-
There are five resonance structures of Phenanthrene, one of which is shown. Draw the otherfour. Phenanthrene
-
What is the speed of a conduction electron whose energy is equal to the Fermi energy E F for, (a) Na, (b) Au, and (c) Sn?
-
For the data in Problem 42, how would you predict demand for medical kits using (a) moving averages and (b) exponential smoothing (with alpha values equal to 0.5 and greater) for the 21st week? Data...
-
For a light ray that crosses the interface between medium 1 having index of refraction \(n_{1}\) and medium 2 having index of refraction \(n_{2}\), what relationship between \(\theta_{1}\) and...
-
The atmosphere of the planet Venus is almost entirely composed of carbon dioxide (about 96.5 % carbon dioxide). The carbon dioxide on Venus might be in equilibrium with carbonate ions in minerals on...
-
Seniority quantum numbers typically measure how many fermions are in some sense "not paired" with another fermion. For the quasispin model of Problem 31.3 , define the Racah seniority $v$ through...
-
(a) Place a perfectly conducting sphere with radius a in a uniform electric field E 0 and let an origin centered electric dipole field represent the field produced by the sphere. Use this information...
-
explain the nature and treatment of taxation in limited company accounts.
-
Identify the most stable compound:
-
The equilibrium constant for the reaction A(g) B(g) is 10. A reaction mixture initially contains [A] = 1.1 M and [B] = 0.0 M. Which statement is true at equilibrium? (a) The reaction mixture...
-
Draw the complete structures of the following peptides: (a) Thr-Phe-Met (b) Serylarginylglycylphenylalanine (c) IMQDK (d) ELVIS
-
Draw the structure of the phenylthiohydantoin derivatives of (a) Alanine (b) Tryptophan (c) Lysine (d) Proline
-
Show the third and fourth steps in the sequencing of oxytocin.
-
What information may an Appeals Officer not consider when reviewing a taxpayer's case? Select one: a. The cost involved for the IRS to hire an expert witness for litigation. b. Litigation hazards...
-
Carla Vista Cart Inc. has the following information for 2026 : The rate of return on assets Carla Vista Cart Inc. is 81.06%17.27%30.58%14.00%
-
A man wishes to borrow 100$ for two years using the sinking fund method. He pays interest annually, at an annual effective interest rate of 5%. Construct a sinking fund schedule if he replaces the...

Study smarter with the SolutionInn App


