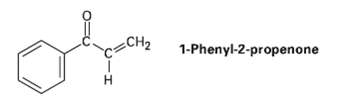
Treatment of 1-phenyl-2-propenone with a strong base such as LDA does not yield an anion, even though
Question:
Treatment of 1-phenyl-2-propenone with a strong base such as LDA does not yield an anion, even though it contains a hydrogen on the carbon atom next to the carbonyl group.Explain.
Transcribed Image Text:
1-Phenyl-2-propenone CH2 H.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (12 reviews)
The illustrated compound 1phenyl2propenone ...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Treating with PGA2 strong base such as sodium tert-butoxide followed by addition of acid converts it to PGC2. Propose a mechanism for this reaction.
-
In the dehydrohalogenation of alkyl halides, a strong base such as tert-butoxide usually gives the best results via the E2 mechanism. (a) Explain why a strong base such as tert-butoxide cannot...
-
(S)-1-Bromo-1,2-diphenylethane reacts with a strong base to produce cis-stilbene and trans-stilbene: a) This reaction is stereo-selective, and the major product is trans-stilbene. Explain why the...
-
The balance sheet of Lamont Bros. follow: (a) What portions of Lamont's assets were provided by debt, contributed capital, and earned capital? Reduce contributed capital by the cost of the treasury...
-
When is the indirect strategy in communicating bad news preferable?
-
How has the development of secondary mortgage markets allowed mortgage issuers to attract additional funds from the capital markets?
-
Verify that each of the terms in the sum-of-squares function (see Equation 9.9 on page 208) Sb y0 y ( y0 Xb ( b0 X0 y b0 X0 Xb is (1 1), justifying writing Sb y0 y ( 2y0 Xb b0 X0 Xb
-
During April, the production department of a process manufacturing system completed a number of units of a product and transferred them to finished goods. Of these transferred units, 37,500 were in...
-
provide a SWOT analysis for apple 1000 words
-
Shauna Coleman is single. She is employed as an architectural designer for Streamline Design (SD). Shauna wanted to determine her taxable income for this year. She correctly calculated her AGI....
-
Base treatment of the following ?, ?-unsaturated carbonyl compound yields an anion by removal of H from the y carbon. Why is hydrogen?s on the y carbon atom acidic? LDA 1:0-I I.
-
When optically active (R)-2-rnethylcyclohexanone is treated with either aqueous base or acid, racemization occurs. Explain.
-
Product A is normally sold for $8.90 per unit. A special price of $6.60 is offered for the export market. The variable production cost is $5.10 per unit. An additional export tariff of 25% of revenue...
-
Repeat Exercise 15 in Chap. 3 to allow the user to enter temperatures for any number of cities using the best iteration structure. Data From Exercise 15 The dew point temperature is a good indicator...
-
Two stacks of positive integers are needed, one containing elements with values less than or equal to 1,000 and the other containing elements with values larger than 1,000. The total number of...
-
Compare Figures 1-2 and 1-12. How do they differ? How are they similar? Explain how Figure 1-12 conveys the idea of speed in development. Figures 1-2 Figures 1-12 Maintenance Planning Implementation...
-
With a neat sketch explain the working of pressure-velocity compounding of impulse steam turbine.
-
The adjusted trial balance for Barry Moving Service as of December 31 is as follows: Required a. Prepare the closing entries at December 31 directly to Retained Earnings in general journal form. b....
-
What are some ways to implement a retrenchment strategy without creating a lot of resentment and conflict with labor unions? AppendixLO1
-
What is the difference between adsorption and absorption?
-
What is the molecular geometry about nitrogen in CH 3 NH 2 ? (a) Trigonal planar (b) Tetrahedral (c) Trigonal pyramidal (d) Bent
-
Within set, rank the compounds in order of increasing rates of their SN2 reactions. Explain your reasoning. 1-bromocyclohexene, bromocyclohexane, 1-(bromomethyl)cyclohexene
-
How many CO ligands would be accommodated by Fe(O) if we assume that the resulting complex follows the 18-electron rule?
-
Give the structure for each of the following compounds. (a) Valerophenone (b) -chlorobutyraldehyde (c) 3-hydroxy-2-butanone (d) 3-cyclohexenone
-
In the Marriott example, one discussion point considered when a firm might use a single hurtle rather than different divisional or business unit rates. When a single rate is used and the divisions...
-
Which of the following are elements of a bootstrappable business model? Indicate ALL that apply. Large up-front capital investment Recurring revenue stream Long sales cycles Word of mouth advertising
-
Hooligan Adventure Supply produces and sells various outdoor equipment. The Molding and Assembly production departments are supported by the Personnel and Maintenance departments. Personnel costs are...

Study smarter with the SolutionInn App


