Use Table 4.2 to predict whether the equilibrium for these reactions favors the reactants or the products.
Question:
Use Table 4.2 to predict whether the equilibrium for these reactions favors the reactants or the products.
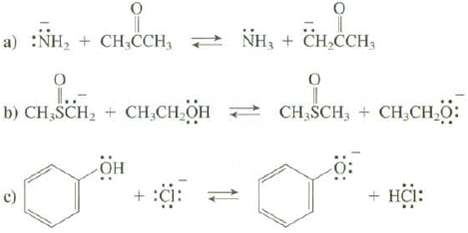
Transcribed Image Text:
a) :NH₂ + CH₂CCH, NH₂ + CH₂CCH, O 1..- b) CH₂SCH₂ + CH₂CH₂OH он + :C: 9 CH SCH, CH₂CH₂O: o + HCI:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 76% (13 reviews)
Remember the equilibrium favors the formation of the weaker acid and the weaker base The ...View the full answer

Answered By

Shashikant Waghule
I worked as Online Tutor in Tecknit IT Enabled Services, Pune.
Currently working as Assistant Professor in Engineering College, but due to COVID-19 situation teaching online to Engineering students, helping students to solve their homework, assignments and mathematical problems.
Helping many students through social media to solve their maths problems.
Please give me chance to share my knowledge with SolutionInn students.
Thanking You.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Use Figure 16.3 to predict whether the equilibrium lies to the right or to the left in the following reactions:
-
Predict whether the following reactions will be spontaneous in acidic solution under standard conditions: (a) Oxidation of Sn to Sn2+ by I2 (to form I-) (b) Reduction of Ni2+ to Ni by I- (to form I2)...
-
Predict the products of the reactions of the following compounds with chromic acid and also with PCC. (a) Cyclohexanol (b) 1-methylcyclohexanol (c) Cyclopentylmethanol (d) Cyclohexanone (e)...
-
Why is it important to have a defined project scope? Why is it important to make sure there is agreement about the scope? Is there anything in the "Why Should You Use the WBS?
-
Mountain View Hospital has adopted a standard costing system for evaluation and control of nursing labour, Diagnosis Related Groups (DRGs) are used as the output measure in the standard costing...
-
Pop Corporation acquired 80 percent of the outstanding stock of Son Corporation for $1,120,000 cash on January 3, 2016, on which date Son's stockholders' equity consisted of capital stock of $800,000...
-
E 5-10 Consolidated income statement (upstream sales) Pop Corporation purchased an 80 percent interest in Son Corporation for $1,200,000 on January 1, 2017, at which time Sons stockholders equity...
-
Globe Corporation, a new environmental control company, initiated a performance based stock option plan for its management on January 1, 2010. The plan provided for the granting of a variable number...
-
What are slipping tasks? Tasks that are over budget Tasks that are risky Tasks that are failing quality control tasks whose finish date is past the baseline finish date
-
24,000 lb/hr of 35API distillate is cooled from 400 to 300F by 50,000 lb/hr of 34API erude oil heated from an inlet temperature of 250F. Pressure drops of 10 psi are allowable, and a dirt factor of...
-
Explain which compound is the weaker base. NH or NH NO b) or
-
Explain whether each of the following solvents would be acceptable for reactions involving this anion: (a) Liquid NH3 (b) CH3CH2OH (c)CH3CH2OCH2CH3 CHC=C: solvent
-
Create two examples, one of a table that is a relation and one of a table that is not a relation.
-
Test the given claim. Assume that a simple random sample is selected from a normally distributed population. Use either the P-value method or the traditional method of testing hypotheses. Company A...
-
Trojan Technologies As Joyce Guo, senior buyer at Trojan Technologies Inc. in London, Ontario, Canada, finished her presentation, Randy Haill, materials manager, Made the following comments to her:...
-
In 2022, Andrew, who is single, has a comfortable salary from his job as well as income from his investment portfolio. However, he is habitually late in filing his federal income tax return. He did...
-
Express the confidence interval (0.045,0.123) in the form of p^ - E < p < p^+ E.
-
Boomtown is preparing a cost analysis of the three departments: Parks. Fire, and Water. To comply with accuracy standards in allocating indirect costs, Boomtown will employ the step-down method of...
-
3.1 Using the information given in exercise 2.5, analyse the transactions in debit/credit form showing, in each case, that the extended accounting equation is maintained, e.g. Deborah purchased a...
-
1) Predict the organicproduct formed when BzCl reacts with cyclohexanol. BzCl = benzoylchloride. 2) Provide the majororganic product of the reaction below. 3) Draw the structureof the product formed...
-
Show that if a < b then a < (a + b)/2 < b.
-
When o-phthalaldehyde is treated with base, o-(hydroxymethyl) benzoic acid is formed. Show the mechanism of this reaction. CO2H 1. "OH 2. * " "CH2 o-Phthalaldehyde o-(Hydroxymethyl)benzoic acid
-
What is the stereochemistry of the pyruvate reduction shown in figure, does NADH lose its pro-R or pro-S hydrogen? Does addition occur to the Si face or Re face of pyruvate?
-
Assign R or S stereochemistry to the two chirality centers in isocitrate, and tell whether OH and H add to the Si face or the Re face of the double bond.
-
Problem 3 Progress Company acquired 6 0 % of Stall Corporation on 1 2 0 2 0 . Fair values of Stall's assets and liabilities approximated book values on that date. Progress uses the initial value...
-
C: The sor at the poopecin 0ieund to twe oxind places)
-
What information may an Appeals Officer not consider when reviewing a taxpayer's case? Select one: a. The cost involved for the IRS to hire an expert witness for litigation. b. Litigation hazards...

Study smarter with the SolutionInn App


