Using the information available in Figure 4.2, predict the position of the equilibrium in these reactions; that
Question:
Using the information available in Figure 4.2, predict the position of the equilibrium in these reactions; that is, predict whether there is a higher concentration of reactants or products present at equilibrium:
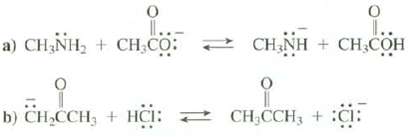
Transcribed Image Text:
0 a) CHÍNH, + CH CO: 1↓ O CH₂NH + CH₂COH 0 b) CH₂CCH₂ + HCI: CH₂CCH₂ + C
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 58% (17 reviews)
The equilibrium favors the formation of the more stable compounds In the ...View the full answer

Answered By

Poonam Chaudhary
I have 15 month+ Teaching Experience
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
A statistics teacher is wondering whether there is a relationship between the marks of students in their first-year business math course, and their marks in the second-year statistics course....
-
Using the information available at the SEC's website or any other authoritative source, describe how the sec is structured.
-
Based on the information available in the case, discuss what you think are the reasons for people's resistance to the MRP implementation. Discuss if there is any value to the task force idea that was...
-
Why is Supplier Relationship is important to a business. explain
-
The 'Real life' on page 504 explains how standard costing systems may be adopted to better suit managers' decision needs. This includes using different definitions of standard costs to suit different...
-
Neilson Tool Corporation's December 31 year-end financial statements contained the following errors: An insurance premium of $66,000 covering the years 2016, 2017, and 2018 was prepaid in 2016, with...
-
E 5-5 Upstream sales Pam Corporation owns an 80 percent interest in Sun Corporation acquired several years ago. Sun regularly sells merchandise to Pam at 125 percent of Suns cost. Gross profit data...
-
Calculate the net present value (NPV) for the following 20-year projects. Comment on the acceptability of each. Assume that the firm has an opportunity cost of 14%. a. Initial cash outlay is $15,000;...
-
17 54. The form of ownership entity for pooled equity investments is heavily dependent on federal tax considerations. Which of the following ownership structures does not provide the benefits of...
-
Consider the choice shown in Problem 3. The probability of a $5 return is 1/2 and of a $12 return is 1/4. How much would these probabilities have to change so that the investor is indifferent between...
-
Indicate whether these species are weaker or stronger bases than hydroxide ion. The Ka is or pKa values are for the conjugate acids. a) :NH, (K = 10-38) c) NH, (PK, = 9.24) b) CHCHCH d) :CI: (pK =...
-
Use the information in Figure 4.2 to predict the positions of the equilibria in the reactions in problem 4.4.
-
Sunburst sells a snowboard, Xpert, that is popular with snowboard enthusiasts. Below is information relating to Sunburst's purchases of Xpert snowboards during September. During the same month, 116...
-
The force vector F has a magnitude of F = 385 lb and acts at point A at an angle 0 = 17 with respect to vertical as shown. The force F is balanced by the tension forces parallel to the two rods AC...
-
D1 Justify the use of a specific moulding technique for the manufacture of a given product
-
the igniter is made of a wire with paper tape holding it . In the head of the igniter is a very thin wire surrounded by pyrotechnic material. Pressing the second switch allows more current to flow...
-
Problem - Process Costing Atticus Electronics produces travel batter pack chargers. The company uses a process costing system. The following information pertains to operations for November Percentage...
-
B . what is the wavespeed? C . What is the frequency? D . What is the wave number? E . At t = 0 . 4 9 s , what is the diplacement of the string at x = 5 . 2 m ?
-
Have you ever known someone who was suicidal? Was appropriate help readily available to this person? Would you have been prepared to refer this person to an appropriate resource?
-
Which of the followingcarbocations is the least stable? CH3CH2 . CH3CHCH3 CH3 I . CH3C0 T CH3 IV. V. CH3 CH3CCH2 CH3
-
Graph the function y = x 3 150x.
-
Draw an energy diagram for a two-step reaction with Keq > 1. Label the overall G, transition states, and intermediate. Is G positive or negative?
-
Draw an energy diagram for a two-step exergonic reaction whose second step is faster than its first step.
-
Draw an energy diagram for a reaction with Keq = 1. What is the value of G in this reaction?
-
On April 1, year 1, Mary borrowed $200,000 to refinance the original mortgage on her principal residence. Mary paid 3 points to reduce her interest rate from 6 percent to 5 percent. The loan is for a...
-
Give a numerical example of: A) Current liabilities. B) Long-term liabilities?
-
Question Wonder Works Pte Ltd ( ' WW ' ) produces ceramic hair curlers to sell to department stores. The production equipment costs WW $ 7 0 , 0 0 0 four years ago. Currently, the net book value...

Study smarter with the SolutionInn App


