Vanillin (the natural vanilla flavoring) occurs in nature as a -glycoside of glucose. Suggest a structure for
Question:
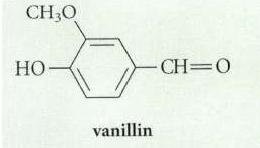
Transcribed Image Text:
CH,O vanillin
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 61% (13 reviews)
Because naturally occurring glycosides generally ha...View the full answer

Answered By

Branice Buyengo Ajevi
I have been teaching for the last 5 years which has strengthened my interaction with students of different level.
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Vanillin is the active component of natural vanilla flavoring. OCH vanillin
-
Pectin, which occurs in plant cell walls, exists in nature as a polymer of D-galacturonic acid methylated at carbon 6 of the monomer. Draw a Haworth projection for a repeating disaccharide unit of...
-
Vanillin, the dominant flavoring in vanilla, contains C, H, and O. When 1.05 g of this substance is completely combusted, 2.43 g of CO2 and 0.50 g of H2O are produced. What is the empirical formula...
-
The adjusted trial balance for Ray Corporation at July 31, 2017, the corporation's fiscal year end, contained the following: Of the lease liability amount, $16,250 is due within the next year. Total...
-
Esther (All) God is not mentioned in this book. How does Gods providence show up in this book?
-
aimer Company s first weekly pay period of the year ends on January 8. On that date, the column totals in Palmers payroll register indicate its sales employees earned $69,490, its office employees...
-
Drafting NFL quarterbacks. The Journal of Productivity Analysis (Vol. 35, 2011) published a study of how successful NFL teams are in drafting productive quarterbacks (see Exercise 1.14, p. 48). The...
-
Central Airlines would like to set up a control chart to monitor its on-time arrival performance. Each day over a 10-day period, Central Airlines chose 30 flights at random and tracked the number of...
-
Rates are 8% p.a. compounded semi-annually. a. What is the value today of a security that promises to pay you $10,000 each two years forever with the first cash flow due in exactly1 year? b. What is...
-
8.1 Create a one-way data table for profit at different levels of supplier contact in range B22:C33. Ensure that the price in cell C3 is $290 and the advertising budget in cell C5 is $35,000,000 (you...
-
Into what other aldose and 2-ketose would each of the following aldoses be transformed on treatment with base? Give the structure and name of the alssose, and the structure of the 2-ketose. D-allose
-
Draw structures for: Is opropy 1-D-galactopyranoside
-
Littles Law is very general, so it is not necessary that flow rate and flow time use the same time units. True or false?
-
As an official sponsor of the Olympics, what specific benefit did John Hancock use to help drive sales in their national offices?
-
assumes that Nia has both a discount rate of zero and faces an interest rate of zero. These assumptions made calculating her constant level of consumption expenditure of $56,000 fairly...
-
Paul Petersen lives in Northern California. He owns a BMW car worth about $20,000. He wants to take a trip to Nevada with his girlfriend Patricia, who lives in Los Angeles. He takes his car into...
-
Do you see gendered patterns of interaction in personal relationships? Does knowing about gender linked patterns affect how other interpret on what happens in a relationships?
-
Significance For bone density scores that are normally distributed with a mean of 0 and a standard deviation of 1, find the percentage of scores that are significantly high (or at least 2 standard...
-
What does the term performance attribution mean?
-
In Problem 8.43, determine the smallest value of for which the rod will not fall out of the pipe. IA -3 in.-
-
Ethylene glycol, HOCH2CH2OH, has zero dipole moment even though carbon-oxygen bonds are strongly polarized. Explain.
-
Make three-dimensional drawings of the following molecules, and predict whether each has a dipole moment. If you expect a dipole moment, show its direction. (a) H2C = CH2 (b) CHC13 (c) CH2C12 (d) H2C...
-
Nitromethane has the structure indicated. Explain why it must have formal charges on N andO. :0: Nitromethane :O:
-
Selected comparative financial statement data for DAS inc. Balance Sheet (En milliers de dollars) 2017 2018 Assets Assets CT - Cash 41.63 47.5 - Accounts Receivable 64.2 72.6 - inventories 969.7...
-
please help!! One chance at turning in!!! 16 rows! I'd highly appreicate it I am unsure what information you need... I provided all Current Attempt in Progress Mike Greenberg opened Grouper Window...
-
Blue Ridge Marketing Inc. manufactures two products, A and B . Presently, the company uses a single plantwide factory overhead rate for allocating overhead to products. However, management is...

Study smarter with the SolutionInn App


