Question: Vapor-liquid equilibrium data at 101.3 kPa are given for the water-formic acid system. From these data, prepare plots like Figures 4.7b and 4.7c. From the
Vapor-liquid equilibrium data at 101.3 kPa are given for the water-formic acid system. From these data, prepare plots like Figures 4.7b and 4.7c. From the plots, determine the azeotropic composition and temperature at 101.3 kPa. Is the azeotrope of the minimum- or maximum-boilingtype?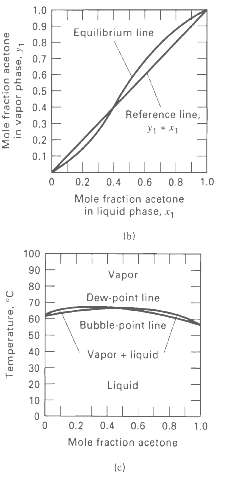
1.0 0.9 Equilibrium line 0.8 0.7 0.6 0.5 D.4 Reference line, 0.3 0.2 0.1 0.2 0.4 0.6 0.8 1.0 Mole fract cn acetone in liquid phase, Ib! 100 90 Vapor 80 Dew-paint line 70 60 Bubble point line 50 40 Vapor + liquid 30 Liquid 0.2 0.4 0.6 0.8 1.0 Mole fraction acetone le) Mole fraction acetone in vapor phase, y1 Temperature, "C
Step by Step Solution
3.35 Rating (167 Votes )
There are 3 Steps involved in it
See plots below From these plots a maximumboiling a... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
37-E-C-E-S-P (103).docx
120 KBs Word File


