Question: Verify ND for unit operation (h) in Table 5.4. How would ND change if a liquid side stream was added to a stage that was
Verify ND for unit operation (h) in Table 5.4. How would ND change if a liquid side stream was added to a stage that was located between the feed stage and stage2?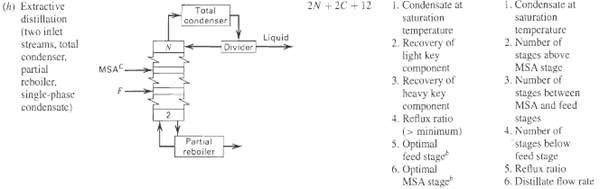
2N + 20 + 12 1. Condensate at 1. Condensate at saturation temperature 2. Number of stages above MSA stage 3. Number of stages between MSA and feed stages 4. Number of stages below (h) Extractive distillation (two inlet streams, total condenser, partial rchoiler, single-phase condensate) Total condenser saturation temperature 2. Recovery of light key component Liquid Divicer MSAC 3. Recovery of heavy key component 4. Reflux ratio 5. Optimal feed stage Partial (ununuu
Step by Step Solution
3.51 Rating (164 Votes )
There are 3 Steps involved in it
Operation h extractive distillation From Eq 570 N R 13 and N A 0 Therefore Eq 1 beco... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
37-E-C-E-S-P (194).docx
120 KBs Word File


