What alkynes would you start with to prepare the followingketones? (a) (b) CHCHH2H CH3CH2CH2CH3
Question:
What alkynes would you start with to prepare the followingketones?
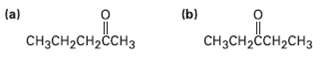
Transcribed Image Text:
(a) (b) CHзCHассH2сHз CH3CH2CH2ČCH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (15 reviews)
a b CH3CHCHCCH H3O HgSO4 OH CH3CH...View the full answer

Answered By

ANDREW KIPRUTO
Academic Writing Expert
I have over 7 years of research and application experience. I am trained and licensed to provide expertise in IT information, computer sciences related topics and other units like chemistry, Business, law, biology, biochemistry, and genetics. I'm a network and IT admin with +8 years of experience in all kind of environments.
I can help you in the following areas:
Networking
- Ethernet, Wireless Airmax and 802.11, fiber networks on GPON/GEPON and WDM
- Protocols and IP Services: VLANs, LACP, ACLs, VPNs, OSPF, BGP, RADIUS, PPPoE, DNS, Proxies, SNMP
- Vendors: MikroTik, Ubiquiti, Cisco, Juniper, HP, Dell, DrayTek, SMC, Zyxel, Furukawa Electric, and many more
- Monitoring Systems: PRTG, Zabbix, Whatsup Gold, TheDude, RRDtoo
Always available for new projects! Contact me for any inquiries
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
What alkenes would you start with to prepare the following alkylhalides? CH-CH (a) (b) Br Br CI (c) (d) CH3CH2CHCH2CH2CH3
-
What alkyne would you start with to prepare each of the following compounds by a hydroboration/oxidationreaction? (b) (a) CH CH-CCHCH3 -CH2CH CH
-
What carbonyl compounds might you start with to prepare the following compounds by Grignard reaction? List all possibilities. (a) 2-Methyl-2-propanol (b) 1-Ethylcyclohexanol (c) 3-Phenyl-3-pentanol...
-
Ancient Indians did not believe in war and violence when it came it an expansion of political territory but relied on peaceful negotiation and strategy. Please explain your views on this quote with...
-
A computer manufacturer sells its laptop model through a web-based distributor, who buys at a unit cost of $200 and sells at a unit price of $500. The product life cycle is so short that the...
-
For very large resistances it is easy to construct R-C circuits that have time constants of several seconds or minutes. How might this fact be used to measure very large resistances, those that are...
-
Describe the type of information that is documented in an issue log. How can you avoid spending too much time documenting and tracking issues? LO.1
-
Newmark & Co. Real Estate, Inc., contacted 2615 East 17 Street Realty, LLC, to lease certain real property on behalf of a client. Newmark e-mailed the landlord a separate agreement for the payment of...
-
Answer one of the questions below: You have 100,000 to set aside for 2 years and so want to invest it in the interim. Would you invest in debt investments or equity investments? What factors would...
-
Black Rose Company has always done some planning for the future, but the company has never prepared a formal budget. Now that the company is growing larger, it is considering preparing a budget....
-
What product would you obtain by hydration of the following alkynes? (a) CH3CH2CH2C=CCH2CH2CH3 CH (b) CCH2CECH-CH2CH3
-
How would you prepare the following carbonyl compounds starting from an alkyne (reddish brown ? Br)? (b) (a)
-
In your own words, define the following terms or symbols: (a) ; (b) ; (c) h; (d) (e) principal quantum number, n.
-
1.A woman eats 65g of protein per day. She weighs 156 lbs. How much protein is she getting per kg of body weight? Explain briefly both question 2.152 lbs = ________________kg. [2.2 lbs = 1 kg] explain
-
The concrete slab shown in Figure is 7m x 5m. The slab is not supported along one of the 7m long edges (free edge). The other three edges are supported and continuous over the supports, and therefore...
-
1. If possible, find 4-B -[{3}][{3}] A=
-
Georgi owns 50% of Forbes, Inc., an S corporation. At the beginning of the current tax year, Georgi had zero basis and an unused net business loss carryover of $10,000. During the tax year, she...
-
luation, of Fundamental Managerial Accounting Concepts. Use Excelshowing all work and formulasto complete the following: Prepare a flexible budget. Compute the sales volume variance and the...
-
Explain the attraction of gaming entertainment to the destination of a tourist. LO.1
-
Baxter, Inc., owns 90 percent of Wisconsin, Inc., and 20 percent of Cleveland Company. Wisconsin, in turn, holds 60 percent of Clevelands outstanding stock. No excess amortization resulted from these...
-
A 25.00-mL sample of an unknown HClO 4 solution requires titration with 22.62 mL of 0.2000 M NaOH to reach the equivalence point. What is the concentration of the unknown HClO 4 solution? The...
-
(a) In most peptides, the amide bonds have the Z conformation; explain why. (b) One particular amino acid residue in the PepC position adopts the E conformation in some cases. Which amino acid...
-
When N - acetyl- L -aspartic acid is treated with acetic anhydride, an optically active compound A, C6H7NO4, is formed" Tleaffient of A with the amino acid L -alanine yields two separable, isomeric...
-
When N - acetyl- L -aspartic acid is treated with acetic anhydride, an optically active compound A, C6H7NO4, is formed" Tleaffient of A with the amino acid L -alanine yields two separable, isomeric...
-
An estimated 84 percent of enterprises now use cloud computing solutions involving multiple clouds, whereas less than 10 percent of large organizations employ just a single public cloud. Group of...
-
XYZ inc. was involved in a tax dispute with the national tax authority. The companys legal counsel estimates that there is a 75% likelihood that the company will lose the dispute and that the amount...
-
3 . Accounting.. How does depreciation impact financial statements, and what are the different methods of depreciation?

Study smarter with the SolutionInn App


