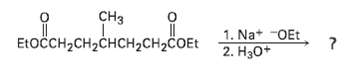
What product would you except from the followingreaction? H ELOCH2CH2CHCH2CH2COET 1. Na+ -OEt 2.
Question:
What product would you except from the followingreaction?
Transcribed Image Text:
сHз ELOČCH2CH2CHCH2CH2COET онро енск до 1. Na+ -OEt 2. Нзо*
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 68% (16 reviews)
OC OEt OEt 1 N...View the full answer

Answered By

Aijaz Khan
I am highly enthusiastic about tutoring. I share a friendly but professional relationship with my students. After completing my electrical engineering I actually taught a course to undergraduates for GATE exam as T.A and it was a brilliant experience and I was one among very few to finish my course on time. I have also helped professors to prepare lessons for my junior fellows while doing my undergrad. Every time I have taught so far, the response has been very heart warming. I hope to continue this and keep on improving it till I am here. Apart from this I have conducted many one on one tutoring lessons.
I believe in focussing on basic and core concepts inorder to keep students' interest alive. My only aim while tutoring is to make student understand concept in such a way that he/she can explain the learnt topic to anyone. Apart from this problem solving is my main focus while tutoring.
I hope to work with SolutionInn for long time. Hope my students will feel the difference.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
What product would you except from the following mixed Claisen-likereaction? Na+ -OCH3 Methanol
-
What enone product would you except from aldol condensation of each of the followingcompounds? (c) CH2H H (b) -CH3
-
What product would you except to obtain from aldol cyclization of hexane-dial, OHCCH 2 CH 2 CH 2 CH 2 CHO?
-
Broadmore Corporation acquired 75 percent of Stem Corporations common stock on January 1, 20X8, for $435,000. At that date, Stem reported common stock outstanding of $300,000 and retained earnings of...
-
The more manageable a firm's culture is, the less valuable it will be for the firm. Agree or disagree-and explain.
-
Although International Shoe manufactured footwear only in St. Louis, Missouri, it sold its products nationwide. It did not have offices or warehouses in the state of Washington, but it did send about...
-
Voluntary Consent. Jerome is an elderly man who lives with his nephew, Philip. Jerome is totally dependent on Philips support. Philip tells Jerome that unless Jerome transfers a tract of land he owns...
-
At the end of its fiscal year, the trial balance for Andy's Cleaners appear as shown below: The following information is also available: a. A study of the company's insurance policies shows that $680...
-
The following information relates to the next three questions: Bond G , described in the exhibit below, is sold for settlement on 1 6 June 2 0 2 0 . The number of days between 1 0 April 2 0 2 0 and 1...
-
Tess is the development manager for the Isabelle Stewart Gardner Museum in Boston. She was in the middle of a large campaign to raise $50 million for a building expansion project. Her development...
-
As shown in figure 23.5, the Claisen reaction is reversible. That is, a ??keto ester can be cleared by base into two fragments. Using curved arrows to indicate electron flow, show the mechanism by...
-
Dieckmann cyclization of diethyl 3-methylheptanedioate gives a mixture of two -keto ester products what are their structures and why is a mixture formed?
-
Sixty-four male students were ordered, after they had violated university alcohol rules, to meet with a school counselor. Borsari and Carey (2005) randomly assigned these students to one of two...
-
1. A corn farmer has observed the following distribution for the number of ears of corn per cornstalk. Ears of Corn Probability 1 2 3 4 .3 .4 .2 .1 Part A: How many ears of corn does he expect on...
-
1. A mass m on a vertical spring with force constant k has an amplitude of A. Using the top of the motion as the origin for both gravitational potential energy and spring potential energy: (a) Find...
-
2. Consider the PDE Utt - Uxx + Ut - Ux = 0 (1) for < < and 0
-
On April 1, 2024, Chardonnay pays an insurance company $12,480 for a two- year fire insurance policy. The entire $12,480 is debited to Prepaid Insurance at the time of the purchase. Record the...
-
Which retailer(s) should represent and sell your product?Why?In terms of their range of distribution coverage, is your retailer intensive, selective and exclusive? Why is this aspect important to...
-
Understand the difference between preventive and corrective discipline. Explain progressive discipline and the importance of managements involvement when it is administered.
-
If someone's Z-score for a variable was 0.67. Their score is a significant extreme score. Their score is not significant. O Their score is slightly above average. O Their score is an outlier.
-
Each compound contains both ionic and covalent bonds. Write ionic Lewis structures for each of them, including the covalent structure for the ion in brackets. Write resonance structures if necessary.
-
SN1 substitution and E1 elimination frequently compete in the same reaction. (a) Propose a mechanism and predict the products for the solvolysis of 1-bromo-1-methylcyclopentane in ethanol. (b)...
-
The solvolysis of 2-bromo-3-methylbutane potentially can give several products, including both E1 and SN1 products from both the unrearranged carbocation and the rearranged carbocation. Mechanisms...
-
Finish Partially Solved Problem 6-1 by showing how the rearranged carbocations give the four products shown in the problem. Be careful when using curved arrows to show deprotonation and or...
-
Assume that the one-year interest rate in the US is 4% and in the Eurozone is 6%. According to interest rate parity (IRP), What should the one-year forward premium or discount of the euro be (use of...
-
Comparative financial statements for Weller Corporation, a merchandising company, for the year ending December 31 appear below. The company did not issue any new common stock during the year. A total...
-
Mrquered Mrquered

Study smarter with the SolutionInn App


