What two constitutional isomers could be formed in the following Diels-Alder reaction? CO,CHs H,C CH2
Question:
What two constitutional isomers could be formed in the following Diels-Alder reaction?
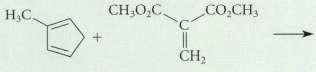
Transcribed Image Text:
CO,CHs H,C CH2
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (7 reviews)
t wo possible orientations of ...View the full answer

Answered By

Nazrin Ziad
I am a post graduate in Zoology with specialization in Entomology.I also have a Bachelor degree in Education.I posess more than 10 years of teaching as well as tutoring experience.I have done a project on histopathological analysis on alcohol treated liver of Albino Mice.
I can deal with every field under Biology from basic to advanced level.I can also guide you for your project works related to biological subjects other than tutoring.You can also seek my help for cracking competitive exams with biology as one of the subjects.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Two constitutional isomers having molecular formula C4H6O are both symmetrical in structure. In their infrared spectra, neither isomer when in dilute solution in CCl4 (used because it is nonpolar)...
-
Two constitutional isomers of molecular formula C8H12O are formed in the following reaction. Ignoring stereochemistry suggest reasonable structures for these Diels-Alder adducts. H.C
-
Compare the following two constitutional isomers. The 13 C NMR spectrum of the first compound exhibits five signals, while the second compound exhibits six signals. Explain. .
-
You borrowed $325000 using a 30- year fixed rate mortgage with a 5.25% interest rate: A) What is your schedule monthly payment? B) What is the amount of interest and principal paid with the first...
-
The following data (in thousands of dollars) have been taken from the accounting records of Karmana Corporation for the just completed year. (This company puts all manufacturing overhead into work in...
-
What is a standard financial analysis plan?
-
_____________ is a quantitative risk analysis tool that uses a model of a system to analyze its expected behavior or performance. a. Simulation b. Sensitivity analysis c. Monte Carlo analysis d. EMV ...
-
On December 31, 2010, Magily Company acquired the following three intangible assets : (a) A trademark for $30,000. The trademark has seven years remaining in its legal life. It is anticipated that...
-
A $513,000 bond issue sold for $486,000. Therefore, the bonds: 7 Multiple Choice 8 00:22:04 Print Sold at a discount because the stated interest rate was higher than the market rate. Sold for the...
-
You are the Owners project manager on an electrical upgrade project. Existing overhead power lines are being buried in a duct bank to hide them from view in an area of the town that is being...
-
What product would be expected from the Diels-Alder reaction of 1, 3-butadiene and ethylene?
-
Complete the following Diesls-Alder reaction NC CN NC CN
-
When Irene Rosenfeld unveiled her turnaround plan, she knew Kraft would need to win back customers both at home and abroad. To concentrate on the companys core brands, Rosenfeld sold off Minute Rice,...
-
what methods ,to do ,or steps do you need to be sure to address when need to make a change in your organization that will help you navigate the organizational culture related to change? Especially...
-
How do you best receive information? Do you prefer written or oral reports? Shorter or longer briefings or reports? Quantitative or qualitative data? Formal or informal styles? How do you ensure...
-
Continuing with an examination of the laws in the state you've written about in earlier discussions, what state and local statutes exist that address the medical conditions or needs of eligible...
-
1. Think about the various soft drinks that you know from the local market and chose any 3 out of that ( e.g. Coca-Cola, Pepsi, 7-Up, Mirinda Citrus, Saudi Champagne, Shaani, Sun Top & Sun Cola,...
-
Leadership is an integral element in any job, regardless of the work title. However, it is important to recognize that leadership is not just one single skill; instead, success in leadership depends...
-
Why is operating indicator analysis important?
-
Using (1) or (2), find L(f) if f(t) if equals: t cos 4t
-
Doreen Dimwhistle has proposed the following variations on the Chichibabin reaction: She is shocked to find that neither of these reactions works as planned and has come to you for an explanation....
-
At 368C the NMR resonances for the ring methyl groups of isopropylmesitylene (protons H a and H b in the following structure) are two singlets at 2.25 and 2.13 with a 2 : 1 intensity ratio,...
-
Draw a variation on the rebound hydroxylation mechanism that accounts for the formation of epoxides from some alkenes by CyP450 as shown in Fig. P17.38. C=C +47 +47 Felv Fell S S. Figure P17.38...
-
nformation pertaining to Noskey Corporation s sales revenue follows: November 2 0 2 1 ( Actual ) December 2 0 2 1 ( Budgeted ) January 2 0 2 2 ( Budgeted ) Cash sales $ 1 0 5 , 0 0 0 $ 1 1 5 , 0 0 0...
-
The management team of Netflix maintains a stable dividend using the Lintner model: Dt+1 = Dt + EPS Target Payout Where Dt (Dt+1) = dividend in the current period t (the next period t + 1) EPSt =...
-
#1 #2 hapter 50 10 D Werences lav Help Required information [The following information applies to the questions displayed below) Archer Company is a wholesaler of custom-built air-conditioning units...

Study smarter with the SolutionInn App


