Within set, which compound should be more reactive in carbonyl-addition reactions? Explain your choices. H,CCC CH or
Question:
Within set, which compound should be more reactive in carbonyl-addition reactions? Explain your choices.
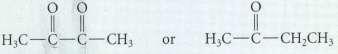
Transcribed Image Text:
H,CCC CH or HCC CH2CH с-с-с
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (10 reviews)
We use the same principles to predict reactivity that we use to predict relativ...View the full answer

Answered By

Shem Ongek
I am a professional who has the highest levels of self-motivation. Additionally, I am always angled at ensuring that my clients get the best of the quality work possible within the deadline. Additionally, I write high quality business papers, generate quality feedback with more focus being on the accounting analysis. I additionally have helped various students here in the past with their research papers which made them move from the C grade to an A-grade. You can trust me 100% with your work and for sure I will handle your papers as if it were my assignment. That is the kind of professionalism that I swore to operate within. I think when rating the quality of my work, 98% of the students I work for always come back with more work which therefore makes me to be just the right person to handle your paper.
4.80+
174+ Reviews
426+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Within each set, which compound should show NMR absorptions with the greater chemical shifts? Explain your choices. (1) (2)
-
Which alkyl halide would you expect to be more reactive in an E2 reaction? a. b. c. d. CH3 CHCHCHCH.CH, or CH.CHCH CHCH. Br Br Br CH3CH2CH CHCH3 or CH3CH2CHCH CH3 CH2CHCH2CH3 or CH2CH CHCH3
-
Which alkyl halide would you expect to be more reactive in an SN2 reaction with a given nucleophile? In each case, you can assume that both alkyl halides have the same stability a. CH3CH2CH2Br or...
-
Which is the most costly option (in terms of impact in other parts of the organization, not absolute dollars)? Which is the least costly?
-
Franco and Elisa share income equally. During the current year the partnership net income was $40,000. Franco made withdrawals of $12,000 and Elisa made withdrawals of $17,000. At the beginning of...
-
The objective of the exercise is to familiarize you with the capabilities of smartphones to identify human activity. The data set is available at archive.ics.uci.edu/ml/datasets/...
-
Apply the ln transformation to the predictor, giving us the transformed predictor variable ln popn. Note that the application of this transformation is due solely to the skewness inherent in the...
-
Killys Baskets has the following current year costs: Variable costs ......... $6 per unit Fixed costs ........... $7,000 Killy and a key supplier have entered into an arrangement that will result in...
-
McGilla Golf is evaluating a new line of golf clubs. The clubs will sell for $1,080 per set and have a variable cost of $490 per set. The company has spent $177,500 for a marketing study that...
-
Garvey Company sells machine parts to industrial equipment manufacturers for an average price of $0.75 per part. There are two types of customers: those who place small, frequent orders and those who...
-
Which carbonyl compound should form the greater proportion of cyanohydrin at equilibrium? Draw the structure of the cyanohydrin, and explain your reasoning. CH-O or CH,CH,CH propanal benzaldehyde
-
Show how ethyl bromide can be used as a starting material in the preparation of each of the following compounds. (a) (b) (CH CH2) CCH ,,
-
Jackson Corporation prepared the following book income statement for its year ended December 31, 2014: Information on equipment depreciation and sale: Equipment 1: Acquired March 3, 2012 for...
-
Analysts and investors often use return on equity ( ROE ) to compare profitability of a company with other firms in the industry. ROE is considered a very important measure, and managers strive to...
-
Provide a brief summary of the case. Respond to the following: 1. Discuss the factors which contributed to the success of the change process in terms of unfreeze, move, and refreeze stages in force...
-
Prepare a proposal where a government agency meets with consumer groups and producers on how to address the shortages in rice, sugar, onions, and fuel, i.e. oil, gasoline and the like. Use the format...
-
Decided to embark on a personal improvement project centered around time management after reviewing the insightful workbook by Neuhauser et al. (2004). My decision was influenced by my recognition...
-
You are the Senior Manager of IAuditYou LLP, you were recently assigned to take over a very important client for the company, The engagement partner, Max Roff, has been the audit partner for the past...
-
A technician supposedly bragged that he earned 25 percent a month for a 10year period using his charts. Assuming he started with a $1,000 investment, determine how much money he would have at the end...
-
(a) What is the focal length of a magnifying glass that gives an angular magnification of 8.0 when the image is at infinity? (b) How far must the object be from the lens?
-
Explain why one of these anions is much more stable than the other: : a) CH-C-CH-CH b) CH CH3 : CH-C-CH-CH CH-C=N:
-
Explain why one of these carbocations is much more stable than the other: + CH-CH3 CH CH
-
Show energy level diagrams for the MOS of these compounds: a) H-C=C-CH3 b) CH0H c) CH3-C-H
-
Simpson Ltd is a small IT company, which has 2 million shares outstanding and a share price of $20 per share. The management of Simpson plans to increase debt and suggests it will generate $3 million...
-
The following are the information of Chun Equipment Company for Year 2 . ( Hint: Some of the items will not appear on either statement, and ending retained earnings must be calculated. ) Salaries...
-
Alta Ski Company's inventory records contained the following information regarding its latest ski model. The company uses a periodic inventory system. Beginning inventory, January 1, 2018 1,250 units...

Study smarter with the SolutionInn App


