Write resonance structures for the followinganions: (a) (b) {c) -, N=CCHCOCH3 CHH CH3CH (e) (d) H
Question:
Write resonance structures for the followinganions:
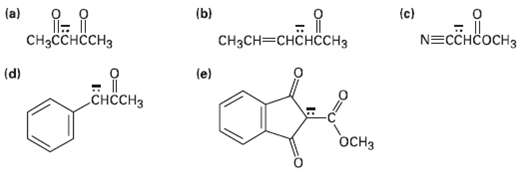
Transcribed Image Text:
(a) (b) {c) Мсбавоь онен-окасен, N=CCHCOCH3 CHасснссHз CH3CH—снснсснз (e) (d) снссHз OCH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 61% (18 reviews)
a b O O tytatytatyta H3C CH3 H c H3C NC H I C 0 CH3 0 O H...View the full answer

Answered By

Carly Cimino
As a tutor, my focus is to help communicate and break down difficult concepts in a way that allows students greater accessibility and comprehension to their course material. I love helping others develop a sense of personal confidence and curiosity, and I'm looking forward to the chance to interact and work with you professionally and better your academic grades.
4.30+
12+ Reviews
21+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Write resonance structures for the arenium ions formed when ethylbenzene reacts with a Br+ ion (as formed from Br2/FeBr3) to produce the following ortho and para products. Br FeBr Br
-
Write resonance structures for the azide ion, N3-. Explain how these resonance structures account for the fact that both bonds of the azide ion have the same length.
-
Write resonance structures for tropylium cation sufficient to show the delocalization of the positive charge over all seven carbons.
-
18. Equal volumes of two solutions containing 3.65g of HCl and 4.0 g of NaOH respectively are mixed. The pli of the mixture is 2) <7 3) >7 19. When Imi of 0.IN HCIIS added to 1 litre of a solution of...
-
What techniques do writers of successful online sales messages use?
-
1. Research AIESEC, the organization Kissam had her internship with, and comment on whether it would be an appropriate way for you to build your global mindset. 2. Kissam stayed abroad when her...
-
Prediction: One use of a fitted regression equation is to predict response-variable values for particular future combinations of explanatory-variable scores. Suppose, therefore, that we fit the model...
-
Marty Kimble, who retired many years ago after winning a huge lottery jackpot, wants to start a new company that will sell authentic sports memorabilia. He plans to name the company Pro Athlete...
-
Prepare the adjusting entry to record cost of goods sold (under FIFO) for 2018 and adjust the inventory account to its year-end balance. How to do the entry on the periodic approach ?? is it correct...
-
Conte Chemical Co. uses the weighted average cost method. All materials are added at the start of the production process. Labor and overhead are added evenly at the same rate throughout the process....
-
Rank the following compounds in order of increasingacidity: (a) CH3CH2CO2H (b) CH3CH2OH (c) (CH3CH2)2NH (d) CH3COCH3 (e) (f) CCI3CO2H CCH-CCH3
-
Predict the product(s) of the followingreactions: (b) 1. Nat "OEt Co (a) 2. CH3I eat (c) Br2, PBr3 H20 CH-CH2C (d) " NaOH, H20 12
-
Ask each student to think about individual family members, friends, and acquaintances. On paper, have them identify the people who act as opinion leaders, product innovators, and market mavens....
-
Implement the nearest neighbor algorithm in the programming language of your choice. The algorithm should work with vectors of up to 10 integer values and allow up to 10 integer classifications. By...
-
Use the operators described in Section 16.2.4 and the STRIPS method to solve the block world planning problem shown in Figure 16.11. The first state shown is the start state and the second state is...
-
Implement a Bayesian belief network in the programming language of your choice to represent a subject in which you are interested (for example, you might use it to diagnose medical conditions from...
-
Researchers have measured the acceleration of racing greyhounds as a function of their speed; a simplified version of their results is shown in Figure P4.67. The acceleration at low speeds is...
-
If the rate at which energy is dissipated by resistor 1 in Figure P31.86 is \(2.5 \mathrm{~W}\), and \(R_{1}=10 \Omega, \mathscr{E}_{1}=12 \mathrm{~V}\), and \(\mathscr{E}_{2}=6 \mathrm{~V},\) (a)...
-
How can an expat program be improved to the benefit of the organization? AppendixLO1
-
Can partitioned join be used for r r.A s? Explain your answer
-
Apply MO theory to determine the bond order in C 2 . a) 0 b) 1 c) 2 d) 3
-
Complete the following reactions. OH NO2 Cr(VI) HSO4
-
Draw the important resonance structures of the radicals formed when each of the following react with R, a general free radical. BHT
-
Give a curved-arrow mechanism for the reaction in Eq. 18.85. Be sure to identify the electrophilic species in the reaction and to show how it is formed. Eq. 18.85 CH3 CH 70% H,504 80 C OH + H2O CH3...
-
You plan to buy a house for $325,000 today. If the house is expected to appreciate in value 8% each year, what will its value be seven years from now?
-
A designated beneficiary of an ABLE account must be ___________ in order to meet the special rules that apply to the increased contribution limit authorized under the Tax Cuts and Jobs Act? a. an...
-
Stans wholesale buys canned tomatoes from canneries and sells them to retail markets Stan uses the perpetual inventory

Study smarter with the SolutionInn App


