2-Chloro-2-mcthylpropane reacts with water in three steps to yield 2-methyl- 2-propanol. The first step is slower than
Question:
2-Chloro-2-mcthylpropane reacts with water in three steps to yield 2-methyl- 2-propanol. The first step is slower than the second, which in turn is much slower than the third. The reaction takes place slowly at room temperature, and the equilibrium constant is near 1.
(a) Give approximate values for ΔG++ and ΔG° that are consistent with the above information.
(b) Draw an energy diagram for the reaction, labeling all points of interest and making sure that the relative energy levels on the diagram are consistent with the information given.
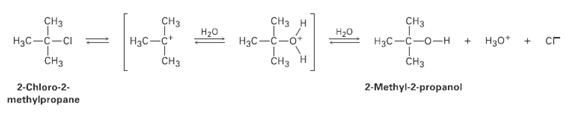 Image caption
Image caption
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





