Question: A blending tank in figure is used to mix a process stream (Stream 1, pure A) with a liquid catalyst (Stream 2, pure B). The
A blending tank in figure is used to mix a process stream (Stream 1, pure A) with a liquid catalyst (Stream 2, pure B). The blending process outlet mixture then flows directly to a reactor. The flow rate of Stream 1 (w1) can vary. It is possible to manipulate the flow rate of Stream 2 (w2) with a control valve, and the outflow also can be manipulated via a control valve in the exit line. The level in the tank is measured. Unfortunately, there is no way of directly measuring the outlet mixture concentration, x. A process control engineer has two options to control the level. In doing this, she hopes indirectly to minimize variations in the ratio of B to A. Method (i): Manipulate the flow rate of Stream 2, W2, while holding the exit flow rate, w constant.
Method (ii): Manipulate the outflow rate, w, while holding w2 constant,
(a) Develop a process model for each of these two cases. Are there enough degrees of freedom to control the level in each case? Which method will keep the B: A ratio more nearly constant for changes in w1?
(b) Because the real objectives are to keep the B: A ratio constant while controlling the level, one would like to be able to measure concentration. If x cannot be measured, is it possible to measure the flow rate of Stream 1 (w1) as well as level (h) and develop a simple feedforward control loop that can help ensure a constant ratio of B: A? If so, what would you manipulate and what model relation(s) would you use?
(c) Are there enough remaining degrees of freedom also to maintain the level approximately constant using this approach? Draw instrumentation diagrams to show how you would implement your control strategies and compare your two-loop control system with Methods (i) and (ii) in terms of its ability to regulate both the concentration of B in the outflow (ratio of B:A) and the level in the blendingprocess.
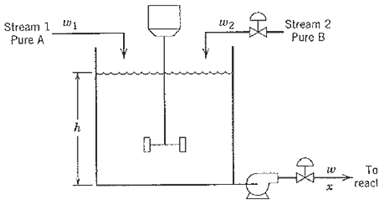
Stream 1 W1 Pure A Stream 2 Pure B To reacl
Step by Step Solution
3.39 Rating (161 Votes )
There are 3 Steps involved in it
Model i Overall mass balance w const... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
38-E-C-E-P-C (19).docx
120 KBs Word File


