CHEMICAL PROCESS PRINCIPLE 2

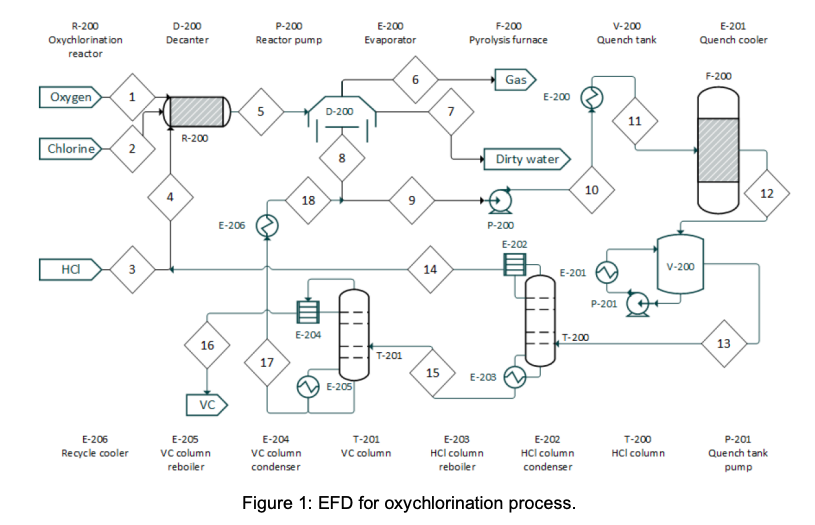
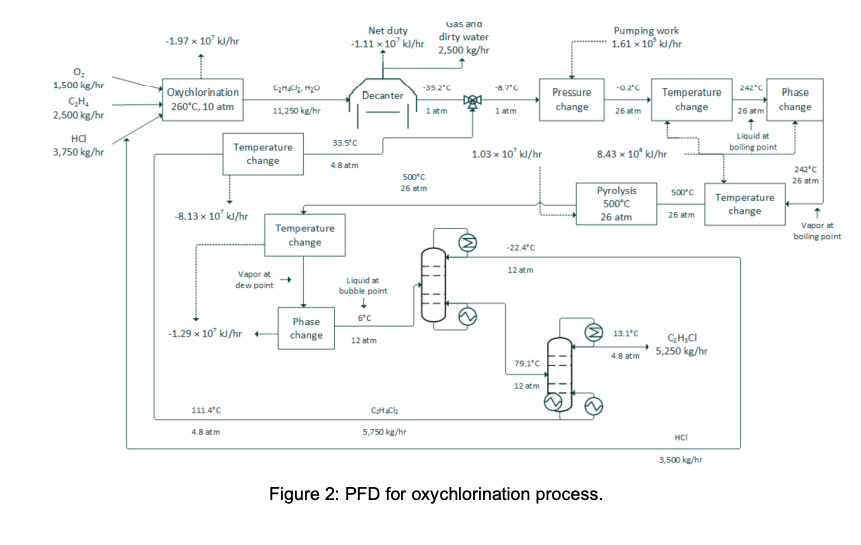
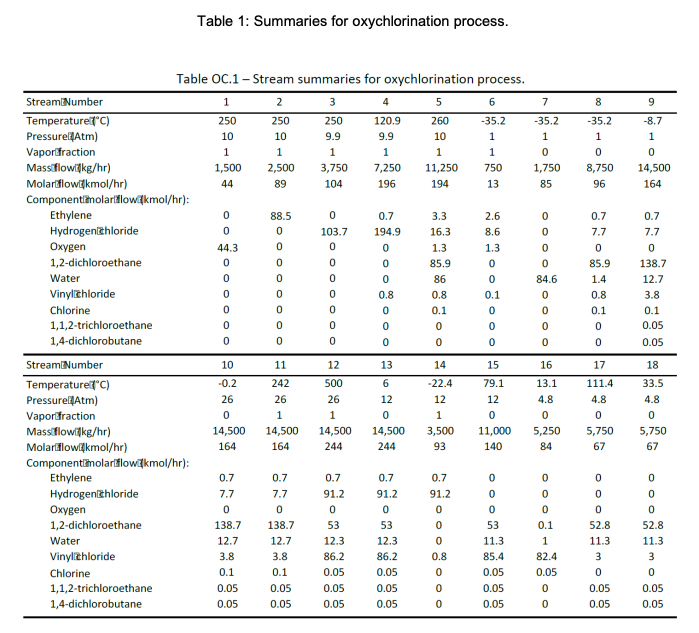
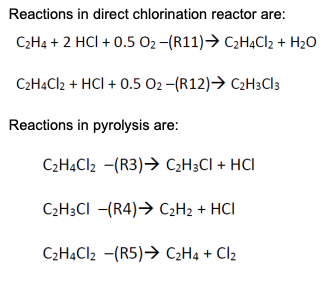

DESCRIPTION The oxychlorination process design is highly comparable to the direct chlorination method, with many shared downstream operations (Benyahia, 2005). It begins with preheated (from storage at 25C) near-stoichiometric feeds of pure ethylene, oxygen, and hydrogen chloride (streams 1,2, and 3; stream 3 joins with a recycle stream to form stream 4) entering an oxychlorination reactor (R-200). Oxychlorination reactors are gas phase reactors, which avoid corrosion by aqueous acid solutions (a classic pitfall of direct chlorination reactors) (Magistro & Cowfer, 1986). To maintain a vapor fraction of 1, the reactor is held at 260C and 10 atm. The oxychlorination products (stream 5) are flown to a decanter (D-200), which removes gas (stream 6) and water (stream 7). The bottoms stream from the decanter, stream 8, is joined by a recycle (to form stream 9) before entering a centrifugal pump and heating system in preparation for pyrolysis (F-200; streams 10 and 11). As with the direct chlorination method, the pyrolysis furnace runs at 500C and 26 atm before opening into a quench tank (V-200; stream 12) at 12 bar and 6C. The quench tank temperature is maintained by continuous material circulation in contact with a refrigerant stream. After cooling, the products travel to distillation columns that are essentially identical to those in the direct chlorination process (T-200 and T-201; streams 13 and 15), which one major deviation. In the oxychlorination model, the distillate HCl from T-200 (stream 14) is recycled upstream to enter the oxychlorination reactor alongside the pure components. Similar to the direct chlorination process, the distillate from T-201 (stream 16) is 98% pure vinyl chloride at 13C and 4.8 atm while the bottoms (stream 17), which is primarily water and 1,2-dichloroethane, is recycled to merge with the enriched 1,2-dichloroethane exiting the bottom of the decanter. The EFD and PFD for oxychlorination process is given in Figure 1 and 2, respectively. Summaries for all the process is given in Table 1. R-200 Oxychlorination D-200 Decanter P-200 Reactor pump E-200 Evaporator F-200 Pyrolysis furnace V-200 Quench tank E-201 Quench cooler reactor 6 Gas F-200 Oxygen 1 E-200 5 D-200 7 7 11 R-200 Chlorine 2. 2. 8 Dirty water 10 4 12 18 9 E-206 P-200 E-202 wo 3 14 V-200 E-201 P-201 E-204 11 T-200 16 13 17 T-201 15 E-205 E-203 VC E-206 Recycle cooler E-205 VC column reboiler E-204 VC column condenser T-201 VC column E-203 HCl column reboiler E-202 HCl column condenser T-200 HCl column P-201 Quench tank pump Figure 1: EFD for oxychlorination process. -1.97 * 10% k/hr Net duty -1.11 x 10' l/hr Gas ana dirty water 2,500 kg/hr Pumping work 1.61 x 109 kJ/hr 35.2C -8.7C O 1,500 kg/hr CH. 2,500 kg/hr -0.2C 242" Oxychlorination 260C, 10 atm Decanter C2HCl, Hio 11,250 kg/hr Phase Pressure change Temperature change 1 atm 1 atm 26 am 26 at change 3,750 kg/hr 33.5C Temperature change Liquid at boiling point 1.03 x 10 kJ/hr 8.43 x 10 kJ/hr 4.8 atm 500C 26 am 242C 26 am 500C Pyrolysis 500C 26 atm Temperature change -8.13 x 10'kJ/hr 26 am Temperature change Vapor at boiling point -22.4C 12 atm Vapor at dew point Liquid at bubble point !!!! 6C -1.29* 10% kJ/hr + Phase change > 12 atm 13.1C GH,CI 48 atm' 5,250 kg/hr 79.1C 12 am 111.4C CH.CH 4.8 atm 5,750 kg/hr HCI 3,500 kg/hr Figure 2: PFD for oxychlorination process. Table 1: Summaries for oxychlorination process. 8 9 -35.2 1 0 8,750 96 -8.7 1 0 14,500 164 194 0 0.7 0.7 Oo Table OC.1 - Stream summaries for oxychlorination process. StreamiNumber 1 2 3 4 5 6 7 Temperature "C) 250 250 250 120.9 260 -35.2 -35.2 PressurelAtm) 10 10 9.9 9.9 10 1 1 Vapor fraction 1 1 1 1 1 1 0 Massiflowkg/hr) 1,500 2,500 3,750 7,250 11,250 750 1,750 Molarilowdkmol/hr) 44 89 104 196 13 85 Componentiamolartflowkmol/hr): Ethylene 0 88.5 0.7 3.3 2.6 Hydrogenlchloride 0 103.7 194.9 16.3 8.6 0 Oxygen 44.3 0 0 1.3 1.3 0 1,2-dichloroethane 0 0 0 85.9 0 0 Water 0 0 86 0 84.6 Vinylizhloride 0 0.8 0.8 0.1 0 Chlorine 0 0 0.1 0 0 1,1,2-trichloroethane 0 0 0 0 0 1,4-dichlorobutane 0 0 0 0 0 0 StreamiNumber 10 11 12 13 14 15 16 Temperature C) -0.2 242 500 6 -22.4 79.1 13.1 Pressurel Atm) 26 26 26 12 12 12 4.8 Vaporfraction 0 1 1 0 1 0 0 Massiflowikg/hr) 14,500 14,500 14,500 14,500 3,500 11,000 5,250 Molartflowikmol/hr) 164 164 244 244 93 140 84 Componentimolartflowikmol/hr): Ethylene 0.7 0.7 0.7 0.7 0.7 0 0 Hydrogenizhloride 7.7 7.7 91.2 91.2 91.2 0 0 Oxygen 0 0 0 0 0 0 0 1,2-dichloroethane 138.7 138.7 53 53 0 53 0.1 Water 12.7 12.7 12.3 12.3 0 11.3 1 Vinylchloride 3.8 3.8 86.2 86.2 0.8 85.4 82.4 Chlorine 0.1 0.1 0.05 0.05 0 0.05 0.05 1,1,2-trichloroethane 0.05 0.05 0.05 0.05 0 0.05 0 1,4-dichlorobutane 0.05 0.05 0.05 0.05 0 0.05 0 OOOOOOO 7.7 0 85.9 1.4 0.8 0.1 0 0 7.7 0 138.7 12.7 3.8 0.1 0.05 0.05 17 18 111.4 4.8 0 5,750 67 33.5 4.8 0 5,750 67 0 OO 0 0 52.8 11.3 3 0 0.05 0 52.8 11.3 3 0 0.05 0.05 0.05 Reactions in direct chlorination reactor are: C2H4 + 2HCl + 0.5 02-(R11) C2H4Cl2 + H2O C2H4Cl2 + HCl + 0.5 02-(R12) C2H3C13 Reactions in pyrolysis are: C2H4Cl2 -(R3) C2H3Cl + HCI C2H3CI -(R4) C2H2 + HCI C2H4Cl2 -(R5) C2H4 + Cl2 YOUR TASK Evaluate on the heat required by one (1) reactive process and one (1) non-reactive process. List all data (such as heat capacity or heat of formation of all components/compounds) and assumptions used to help you with the evaluation and provide the references (whenever possible). Also, derive a differential balance on total mass and vinyl chloride if Stream 16 is to be stored in a well-mixed tank. Provide your initial conditions, Predict volume of the tank to be installed if the tank requires 8 hours to be fully filled












