A former theological student, Heavn Hardley, has turned to chemistry and, during his eighth year of graduate
Question:
A former theological student, Heavn Hardley, has turned to chemistry and, during his eighth year of graduate study, has carried out the following reaction:

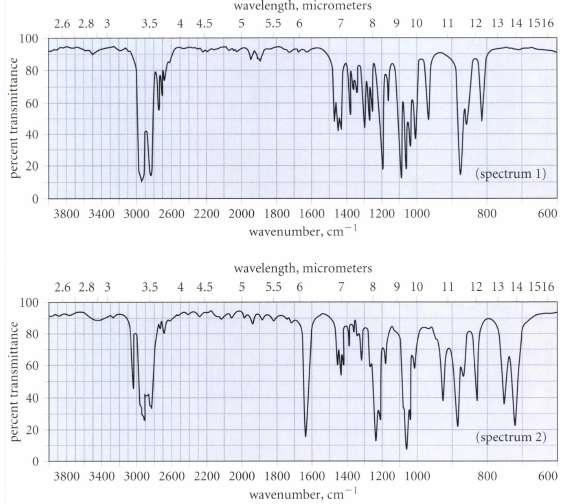
Unfortunately, Hardley thinks he may have mislabeled his samples of A and 5, but has wisely decided to take an IR spectrum of each sample. The spectra are reproduced in Fig. P12.27 on p. 574. Which sample goes with which spectrum? How do you know?
Fig. P12.27
Transcribed Image Text:
Hz,catalyst wavelength, micrometers 2.6 2.8 3 3.5 4 4.55 5.5 6789 10 12 13 14 1516 100 E 80 60 C 40 20 (spectrum 1) 3800 3400 3000 2600 2200 2000 1800 1600 1400 1200 1000 800 600 wavenumber, cm1 wavelength, micrometers 2.6 2.8 3 3.5 4.5 55.5 6 78 9 10 12 13 14 1516 100 80 E 60 ะ 40 o 20 0 (spectrum 2) 3800 3400 3000 2600 2200 2000 1800 1600 1400 1200 1000 800 600 wavenumber, cm
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (12 reviews)
Only spectrum 2 has the C C stretching absorption ...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
A student carried out the following procedure to measure the pressure of carbon dioxide in a soft drink bottle. First, she weighed the bottle (853.5 g). Next, she carefully removed the cap to let the...
-
The following reaction is carried out at 500 K in a container equipped with a movable piston. After the reaction has reached equilibrium, the container has the composition depicted here. Suppose the...
-
A car owner who knows no chemistry has to put antifreeze in his car's radiator. The instructions recommend a mixture of 30% ethylene glycol and 70% water. Thinking he will improve his protection he...
-
The most common way of calculating finance charges is not the simplified one we used but rather the average daily balance. With this method, we calculate the account balance at the end of each day of...
-
Assume that for a 5-year period, large-company stocks had annual rates of return of 21.54 percent, -9.20 percent, -11.99 percent, -21.60 percent, and 29.39 percent. What is the variance of these...
-
Determine how much income Kiera and Logan have per month. Kiera and Logan sit down to make their budget. Kiera works full time as a mental health counselor and sells kids toys on her own. Logan works...
-
Identify the range of customer service calls that should be considered outliers, using: a. the Z-score method; b. the IQR method.
-
In recent years the fast-food chain Wendys International has purchased many treasury shares. From December 28, 2003, to December 31, 2006, the number of shares outstanding has fallen from 115 million...
-
Break-Even Units, Contribution Margin Ratio, Multiple-Product Breakeven, Margin of Safety, Degree of Operating Leverage Jellico Inc.'s projected operating income (based on sales of 450,000 units) for...
-
Given the following sketches, generate an Excel spreadsheet: 1) Count the total degrees of freedom in the sketch. 2) Count the constraints 3) Provide the number of dimensions that are necessary to...
-
Indicate how you would carry out each of the following chemical transformations. What are some of the changes in the infrared spectrum that could be used to indicate whether the reaction has...
-
Arrange the following bonds in order of increasing stretching frequencies, and explain your reasoning. C=C C=C C=0 C-C
-
A Carnot heat engine between heat reservoirs at 550 C and 50 C delivers 420 kJ. Determine the heat transfer from the high-temperature reservoir (kJ) and the engine efficiency. Also determine the...
-
Melannie Inc. sold $8,200 worth of merchandise on June 1, 2015 on credit. After inspecting the inventory, the customer determined that 10% of the items were defective and returned them to Melannie...
-
2. (20 marks) A firm wishes to produce a single product at one or more locations so that the total monthly cost is minimized subject to demand being satisfied. At each location there is a fixed...
-
Evaluate your own negotiation way. Do you have one? how you consider having an excellent negotiaiton skill could help any business person to achieve its goals.
-
j. Interest was accrued on the note receivable received on October 17 ($100,000, 90-day, 9% note). Assume 360 days per year. Date Description Dec. 31 Interest Receivable Interest Revenue Debit Credit
-
A Chief Risk Officer (CRO) is interested in understanding how employees can benefit from AI assistants in a way that reduces risk. How do you respond
-
Would it ever make sense to lease an asset that has a negative NAL when evaluated by a conventional lease analysis? Explain your answer.
-
Revol Industries manufactures plastic bottles for the food industry. On average, Revol pays $76 per ton for its plastics. Revol's waste-disposal company has increased its waste-disposal charge to $57...
-
Draw the structures and give the names of all the dicarboxylic acids with the formula C 6 H 10 O 4 . Indicate which are chiral, which would readily form cyclic anhydrides on heating, and which would...
-
Draw the structure of the major species present in solution when 0.01 mole of the following acid in aqueous solution is treated with 0.01 mole of NaOH. Explain. O Cl O i OH HO-C-C-CH-CH-C-OH T CI
-
Give the product expected when butyric acid (or other compound indicated) reacts with each of the following reagents. (a) Ethanol (solvent), H 2 SO 4 catalyst (b) Aqueous NaOH solution (c) LiAlH 4...
-
If the auditor believes that the financial statements prepared on the basis of the entity's income tax are not adequately titled, the auditor should : A)Issue a resignation of opinion. B)Explain the...
-
initial stock offering to the public. This REIT specializes in the acquisition and management of warehouses. Your firm, Blue Street Advisors, is an investment management company that is considering...
-
Question 3 You have been hired to run a pension fund for Mackay Inc, a small manufacturing firm. The firm currently has Gh5 million in the fund and expects to have cash inflows of $2 million a year...

Study smarter with the SolutionInn App


