Question: A fractionation column operating at 101 kPa is to separate 30 kgh of a solution of benzene and toluene containing 0.6 mass-fraction toluene into an
A fractionation column operating at 101 kPa is to separate 30 kgh of a solution of benzene and toluene containing 0.6 mass-fraction toluene into an overhead product containing 0.97 mass-fraction benzene and a bottoms product containing 0.98 mass-fraction toluene. A reflux ratio of 3.5 is to be used. The r feed is liquid at its boiling point, feed is to the optimal tray, and the reflux is at saturation temperature.
(a) Determine the quantity of top and bottom products
(b) Determine the number of stagesrequired.
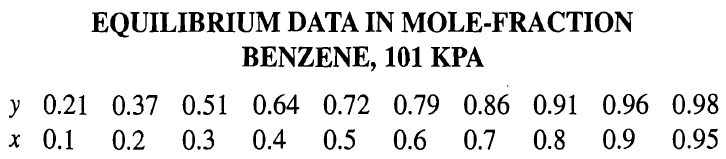
EQUILIBRIUM DATA IN MOLE-FRACTION BENZENE, 101 KPA y 0.21 0.37 0.51 0.64 0.72 0.79 0.86 0.91 0.96 0.98 x 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 0.95
Step by Step Solution
3.45 Rating (177 Votes )
There are 3 Steps involved in it
a First solve the material balance in mass units Then convert to moles and mole fractions so that th... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
37-E-C-E-S-P (257).docx
120 KBs Word File


