(a) Is sodium hydroxide a strong enough base to completely remove a proton from the ?-carbon acetone;...
Question:
(a) Is sodium hydroxide a strong enough base to completely remove a proton from the ?-carbon acetone; that is, does this equilibrium lie nearly completely to the right when sodium hydroxide is the base?
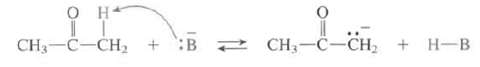
(b) Which common bases can be used to completely remove a proton from acetone?
Transcribed Image Text:
CH3-C-CH2 + :B 2 CH;-C-CH, + H-B
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (10 reviews)
a The equilibrium does not lie completely to the right ...View the full answer

Answered By

Uttam Singh Bhadauriya
I've tutored high school students during my graduation years, and taught them Precalculus and Algebra for college.
I've also taught Calculus and Linear Algebra to College students.
I'm a graduate in Computer Science, but I'm highly enthusiastic about learning and teaching mathematics.
I've qualified exams like IIT-JAM and TIFR, which are entrance exams for research institutes in India.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Identify which of the following bases can be used to deprotonate a terminal alkyne: (a) NaOCH 3 (b) NaH (c) BuLi (d) NaOH (e) NaNH 2
-
A precipitate forms when a small amount of sodium hydroxide is added to a solution of aluminum sulfate. This precipitate dissolves when more sodium hydroxide is added. Explain what is happening.
-
Sodium hydroxide is hygroscopic-that is, it absorbs moisture when exposed to the atmosphere. A student placed a pellet of NaOH on a watch glass. A few days later, she noticed that the pellet was...
-
9. What is the coordination entity formed when excess of aqueous KCN is added to an aqueous solution of copper sulphate? Why is it that no precipitate of copper sulphide is obtained when H2S(g) is...
-
Should Wagner be held responsible for these problems? Explain.
-
An investment club has set a goal of earning 15% on the money they invest in stocks. The members are considering purchasing three possible stocks, with their cost per share (in dollars) and their...
-
A hotel had a problem with people reserving rooms for a weekend and then not honoring their reservations (no-shows). As a result, the hotel developed a new reservation and deposit plan that it hoped...
-
On January 1, 2014, Wolf Creek Country Club purchased a new riding mower for $15,000. The mower is expected to have a 10-year life with a $1,000 salvage value . What journal entry would Wolf Creek...
-
rdings Which of the following is the correct example of a narrative style citation for a quote? Smith (2023, p. 503) postulated "environmental factors have a significant impact on crime commission"....
-
You are the accountant at Indigo. Refer to Appendix III for Indigos Consolidated Balance Sheet as at March 31, 2018. Your manager has assigned you the following tasks. a. Calculate the current ratio,...
-
Explain which the most acidic hydrogen's in these compoundsare c) CH,CCH,CH, b) PHCH CCH3
-
Provide names for thesecompounds: b) a) , CI Br - d) ) e) CH3 NO,
-
In Exercises 11 through 18, either find a number k such that the given function is a probability density function or explain why no such number exists. f(x) = [x+kx for 0x2 0 otherwise
-
Carol's Cupcakes has grown from a home business into a one of the largest event and wedding catering companies in the area. Its founder, Carol Thompson, first dreamed of owning her own company while...
-
Many things have changed for businesses in 2022. The previous 2 business years of 2020 and 2021 have tested businesses and the workforce like nothing else. Not only were profits reduced, and...
-
1) Virginia Tech's motto is "Ut Prosim" which means 'That I May Serve'. Share how you contribute to a community that is important to you. How long have you been involved? What have you learned and...
-
Person Is Arianna Grande Answer all questions Who are they? How successful are they? Why would companies be interested in partnering with them? Identify one company from their industry that you feel...
-
Imagine you have just retired after a long and very successful career (as a physiotherapist). Congratulations! You've made such an impact in the world that business and community leaders from around...
-
Red Bull purchased the New York franchise in Major League Soccer (MLS) in 2009. Why would it have done this?
-
A horizontal annulus with inside and outside diameters of 8 and 10 cm, respectively, contains liquid water. The inside and outside surfaces are maintained at 40 and 20oC, respectively. Calculate the...
-
Of the nearly 1,300 species officially classified as endangered under the 1973 Endangered Species Act, between 450 and 500 are usually considered to be stable or improving. The remaining species are...
-
The following model is that of cholic acid, a constituent of human bile. Locate the three hydroxyl groups, and identify each as axial or equatorial, is cholic acid an A t trans steroid or an A-B...
-
Propose a biosynthetic pathway for the sesquiterpene helminthogermacrene from farnesvldiphosphate.
-
Identify the following fatty acid, and tell whether it is more likely to be found in peanut oil or in redmeat:
-
On April 1, year 1, Mary borrowed $200,000 to refinance the original mortgage on her principal residence. Mary paid 3 points to reduce her interest rate from 6 percent to 5 percent. The loan is for a...
-
Give a numerical example of: A) Current liabilities. B) Long-term liabilities?
-
Question Wonder Works Pte Ltd ( ' WW ' ) produces ceramic hair curlers to sell to department stores. The production equipment costs WW $ 7 0 , 0 0 0 four years ago. Currently, the net book value...

Study smarter with the SolutionInn App


