Explain which the most acidic hydrogen's in these compoundsare c) CH,CCH,CH, b) PHCH CCH3
Question:
Explain which the most acidic hydrogen's in these compoundsare
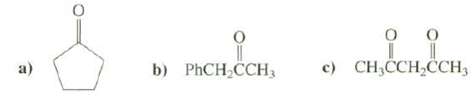
Transcribed Image Text:
c) CH,CCH,ČCH, b) PHCH CCH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 78% (14 reviews)
a The circled hydrogens are more acidic because the conjugate base is stab...View the full answer

Answered By

Jayshree Rathi
Hello Students!
This is Jayshree Rathi. I work on a number of renowned student-centric channels such as Chegg, coursehero, as a certified private tutor.
If you are looking for relevant and original content to complete your assignments, essays, and homework, then contact me and within the promised time, I will deliver you your personalized academic work and help you score the best.
4.80+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Which is the most acidic hydrogen in CH3CH2C = CH?
-
Which is the most acidic hydrogen in each of these compounds? (a) H 2 NCH 2 CH 2 OH (b) CH 3 CH 2 OH (c) CH 3 SH
-
Identify the most acidic hydrogen in each of these compounds: a) HOCCH,CH,SOH 0 CO H 0 I e) CHCCHCOCHCH3 0 b) CH-CH,CH,C=N d) 0 CHOH f) HNCHCOH
-
1. Let f(x) = 2x+6 if x < -1 3x+2 if x -1 (a) What is lim, -1- f(x)? Your answer will depend on b. (b) What is lim,-1+ f(x)? (c) For what values of b does limx-1 f(x) exist? 2. Suppose that f and g...
-
When Theo Chocolate first started is production, the company offered an exotic line of dark-chocolate and milk-chocolate bar and truckles. These early treats had unusual names such as the 3400...
-
A clothing manufacturer has factories in Atlanta, Chicago, and New York. Sales (in thousands) during the first quarter are summarized in the matrix below. During this period the selling price of a...
-
Does the time of day during which one works affect job satisfaction? A study in the Jo~~rnul of Occupational Psychology (Sept. 1991) examined differences in job satisfaction between day-shift and...
-
Reconstructing transactions involving short-term securities available for sale. During 2008, Zeff Corporation sold marketable securities for $14,000 that had a carrying value of $13,000 at the time...
-
Required information [ The following information applies to the questions displayed below ] Now that operations for outdoor clinics and TEAM events are running smoothly, Suzie thinks of another area...
-
Consider the two tables T1 and T2 shown in Figure 8.15. Show the results of the following operations: a. T1 T1.P = T2.A T2 b. T1 T1.Q = T2.B T2 c. T1 T1.P = T2.A T2 d. T1 T1.Q = T2.B T2 e. T1 T2 f....
-
Draw the structures for these compounds: (a) (Z)-Oct-3-en-2-one (b) 3-Ethylheptanal (c) 2, 4-Pentadienal (d) 3, 4-Dimethylbenzaldehyde (e) 1-Phenyl-1-propanone (f) 2, 2, 6, 6-Ttramythleyelohexanone
-
(a) Is sodium hydroxide a strong enough base to completely remove a proton from the ?-carbon acetone; that is, does this equilibrium lie nearly completely to the right when sodium hydroxide is the...
-
What migration paths does WAP 1.x offer for Internet and telephony applications and their protocols? Compare with WAP 2.0.
-
The COVID pandemic has created a crisis for many restaurateurs. The author of one of this week's readings has a suggestion that he thinks could help restaurants survive the crisis. Read the article...
-
Evidence is used to make a decision whenever the decision follows directly from the evidence (Tingling & Brydon, 2010). This is where so many people get it wrong or going by their personal beliefs or...
-
Pick 2 countries, find the price of a Big Mac in each country (if you want to pick another good/service, go ahead), express the price in the local currency, then with the help of exchange rate,...
-
Your task is to educate the public about the role of the Fed in the economy. Role: You are an economic issues reporter for PBS. Audience: Television audience of The Newshour on PBS Situation: Your...
-
Trade Queens Limited is a highly successful FMCG in Zambia. Salient points from the Year-end report indicate the following: Operating profit for the 2022 financial year is up 60% year on year,...
-
Why do you think Red Bull sponsors both events and athletes?
-
A 20-cm-square vertical plate is heated to a temperature of 30oC and submerged in glycerin at 10oC. Calculate the heat lost from both sides of the plate.
-
Under the European Unions Common Fisheries Policy, countries are allocated quotas for the amounts of fish that their fishermen can catch in various areas of the sea. In most European nations,...
-
Draw the following molecules in chair conformations, and tell whether the ring sub-stituents are axial orequatorial: CH (b) (a) CH
-
Lithocholic acid is an A-B cis steroid found in human bile. Draw Lithocholic acid showing chair conformations as in figure, and tell whether the hydroxyl group at C3 is axialequatorial. An A-B trans...
-
Compare the structures of lanosterol and cholesterol, and catalog the changes needed for the transformation.
-
Kenneth lived in his home for the entire year except for when he rented his home (near a very nice ski resort) to a married couple for 14 days in December. The couple paid Kenneth $14,000 in rent for...
-
On December 31, 2021, Shack Store Inc had 143 million shares outstanding, which traded for $643.29 per share. On January 02, 2022, the CEO announced a 20-for-1 stock split. Every shareholder would...
-
o1s= secom o1s= secom

Study smarter with the SolutionInn App


