Question: A mixture that contains 46 wt% acetone (CH3COCH3), 27% acetic acid (CH3COOH), and 27% acetic anhydride [(CH3CO) 2O] is distilled at P = 1 atm.
A mixture that contains 46 wt% acetone (CH3COCH3), 27% acetic acid (CH3COOH), and 27% acetic anhydride [(CH3CO) 2O] is distilled at P = 1 atm. The feed enters the distillation column at T = 348 K at a rate of 15,000 kg/h. The distillate (overhead product) is essentially pure acetone, and the bottoms product contains 1% of the acetone in the feed. The vapor effluent from the top of the column enters a condenser at 329 K and emerges as a liquid at 303 K. Half of the condensate is withdrawn as the overhead product, and the remainder is refluxed back to the column. The liquid leaving the bottom of the column goes into a steam-heated re-boiler, in which it is partially vaporized. The vapor leaving the re-boiler is returned to the column at a temperature of 398 K, and the residual liquid, also at 398 K, constitutes the bottoms product. A flowchart of the process and thermodynamic data for the process materials follow.
(a) Calculate the molar flow rates and compositions of the product streams.
(b) Calculate the condenser cooling requirement Qc (kJ/h).
(c) Use an overall energy balance to determine the re-boiler heating requirement Qr (kJ/h.
(d) If the re-boiler heat is provided by the condensation of saturated steam at 10 bar gauge, at what rate must steam befed?
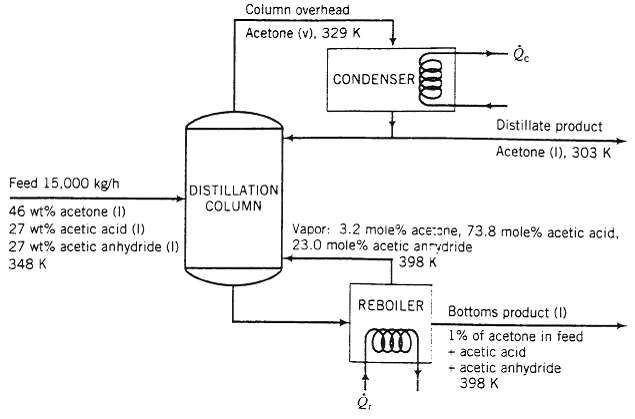
Feed 15.000 kg/h 46 wt% acetone (1) 27 wt% acetic acid (1) 27 wt% acetic anhydride (1) 348 K Column overhead Acetone (v). 329 K DISTILLATION COLUMN CONDENSER (0000) REBOILER (11) lc Vapor: 3.2 mole% ace:one, 73.8 mole% acetic acid. 23.0 mole% acetic anrydride 398 K Distillate product Acetone (1), 303 K - Bottoms product (1) 1% of acetone in feed acetic acid +acetic anhydride 398 K
Step by Step Solution
3.50 Rating (167 Votes )
There are 3 Steps involved in it
Basis 15000 kg feedh 15000 kgh 046 A 027 B 027 C 348 K 1 atm A acetone B acetic acid C acetic anhydr... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
13-E-C-E-C-P (467).docx
120 KBs Word File


