Almost all stable organic species have tetravalent carbon atoms, but species with trivalent carbon atoms also exist.
Question:
Almost all stable organic species have tetravalent carbon atoms, but species with trivalent carbon atoms also exist. Carbocations are one such class of compounds.
(a) How many valence electrons do the positively charged carbon atoms have?
(b) What hybridization do you expect this carbon atom to have?
(c) What geometry is the carbocation likely tohave?
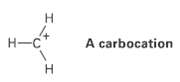
Transcribed Image Text:
H. Н-С A carbocation
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 61% (21 reviews)
a The positively charged carbon atom is surrounded by ...View the full answer

Answered By

Hassan Ali
I am an electrical engineer with Master in Management (Engineering). I have been teaching for more than 10years and still helping a a lot of students online and in person. In addition to that, I not only have theoretical experience but also have practical experience by working on different managerial positions in different companies. Now I am running my own company successfully which I launched in 2019. I can provide complete guidance in the following fields. System engineering management, research and lab reports, power transmission, utilisation and distribution, generators and motors, organizational behaviour, essay writing, general management, digital system design, control system, business and leadership.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
How many valence electrons do each of the following elements have, and what are the specific valence electrons for each element? a. Ca b. O c. element 117 d. In e. Ar f. Bi
-
How many valence electrons does carbon have?
-
How many valence electrons does each of the following atoms have? (a) Na (b) Cl (c) Si (d) B (e) Ne (f) N
-
We often speak of how price rations goods. What are other rationing measures in clinics in which free care is provided?
-
On January 1, 2007, Stephanie Bridges acquired depreciable real property for $50,000. She used straight-line depreciation to compute the asset's cost recovery. The asset was sold for $96,000 on...
-
An extrusion die is used to produce aluminum rods. The diameter of the rods is a critical quality characteristic. The following table shows x and r values for 20 samples of five rods each....
-
how researchers used confidence intervals to study Uber and traffic fatalities
-
Ann deposits $100 at the end of each month into her bank savings account. The bank paid 6% nominal interest, compounded and paid quarterly. No interest was paid on money not in the account for the...
-
Austen Industries manufactures a popular interactive stuffed animal for children that requires four computer chips inside each toy. The company pays $2 for each computer chip. To help to guard...
-
Complete Alvins Music Inc.s (AMI) 2020 Form 1120, Schedule D, and Schedule G (if applicable) using the information provided below. Neither Form 4562 for depreciation nor Form 4797 for the sale of...
-
Complete the electron-dot structure of caffeine, showing all lone-pair electrons, and identify the hybridization of the indicatedatoms. CH H3C. -- Caffeine 0= C
-
A carbanion is a species that contains a negatively charged, trivalent carbon. (a) What is the electronic relationship between a carbanion and a trivalent nitrogen compound such as NH3? (b) How many...
-
A 500-kg load hangs from three cables of equal length that are anchored at the points (-2, 0, 0), (1, 13 , 0), and (1, -3, 0). The load is located at (0, 0, -23). Find the vectors describing the...
-
Units processed during September for material and conversion. Ask an instructor lock lock lock A 3 A copy Determine the cost per equivalent unit for material and conversion cost combined. copy...
-
12% of all college students volunteer their time. Is the percentage of college students who are volunteers different for students receiving financial aid? Of the 338 randomly selected students who...
-
Mervon Company has two operating departments: mixing and bottling. Mixing has 3 3 0 employees and Bottling has 2 2 0 employees. Indirect factory costs include administrative costs of $ 1 8 2 , 0 0 0...
-
XP Ltd. is a manufacturing company with high stock requirements. Management are currently considering their stockholding policy. The following information is available for one stock item, material...
-
Process Costing: weighted average method Required: make a cost of production report in good form. Cost of Production Report-Weighted Average First Dept- Gem Company applies 100% of materials at the...
-
Which description ad goes against which learning style? Honey and Mumford have developed a learning cycle where people learn from their experiences and plan the next step from this learning. They...
-
What are the principal alloying elements in SAE 4340 steel?
-
Consider the truncated virial equation of state where B(T) is the second virial coefficient. Obtain the constraint on B(T) if the fluid is to be thermodynamically stable. PV RT = 1+ B(T) V
-
Show the conjugate bases of these species: a) H-0-H_bH-0 H CH-N-H d) H-C-C-H H
-
Complete these acid-base equations. Use the curved arrow method to show the electron movement in the reactions. Base Acid a) NH, + H: b) CHO + HO: Conjugate acid Conjugate base
-
Indicate whether each of these species is a Lewis acid, a Lewis base, or both: H 1. a) H-C I H d) CH3-N-H T H b) H-O-H :8-3-6: :ci: e) :CI-AI :CI: H c) H-B 1 H
-
Just work out the assignment on your own sheet, you dont need the excel worksheet. Classic Coffee Company Best friends, Nathan and Cody, decided to start their own business which would bring great...
-
Financial information related to the proprietorship of Ebony Interiors for February and March 2019 is as follows: February 29, 2019 March 31, 2019 Accounts payable $310,000 $400,000 Accounts...
-
(b) The directors of Maureen Company are considering two mutually exclusive investment projects. Both projects concern the purchase of a new plant. The following data are available for each project...

Study smarter with the SolutionInn App


