Complete the electron-dot structure of caffeine, showing all lone-pair electrons, and identify the hybridization of the indicatedatoms.
Question:
Complete the electron-dot structure of caffeine, showing all lone-pair electrons, and identify the hybridization of the indicatedatoms.
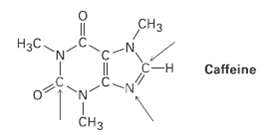
Transcribed Image Text:
CHз H3C. -- Caffeine 0= Cнз
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 72% (18 reviews)
HC 0 0 CH3 CH3 ...View the full answer

Answered By

Deepak singh
I have completed my graduation from computer science engineering . I have good knowledge about programming language and love to solve many codes of any language. I have been an expert to other platforms too and have good experience of giving solution to the students of subject .
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Identify all nonbonding lone pairs of electron in the following molecules, and tell what geometry you expect for each pf the indicated atoms. (a) The oxygen atom in the dimethyl ether, CH3 ? O ? CH3...
-
The file Caffeine contains the caffeine content (in milli-grams per ounce) for a sample of 26 energy drinks: a. Construct an ordered array. b. Construct a stem- and- leaf display. c. Does the ordered...
-
Identify the expected hybridization state and geometry for the central atom in each of the following compounds: a. b. c. d. e. H. I-z: I-
-
Suppose that Marthas income rises to $42,000 per year, and that she increases her consumption of health care visits by fi ve visits. Using the graphs for Exercise 1, draw the new equilibrium. What is...
-
Dedriea contributes to her wholly owned corporation some tangible personal property that she had used in her sole proprietorship business and depreciated. She had acquired the property for $566,000...
-
What categories of ambiguous expressions should be avoided because they could confuse readers for whom English is not a first language?
-
how a researcher used confidence intervals to study popular films
-
Following are income statements for Hossa Corporation for 2017 and 2016. Percentage of sales amounts are also shown for each operating expense item. Hossas income tax rate was 38% in 2016 and 40% in...
-
accouting On September 12. Ryan Company sold merchandise in the amount of $8,400 to Johnson Company with credit terms of 2/0, 30. The cost of the items sold 35,300. Hyan use the periodic inventory...
-
There is a lottery with n coupons and n people take part in it. Each person picks exactly one coupon. Coupons are numbered consecutively from 1 to n, n being the maximum ticket number. The winner of...
-
Allene (see problem 1.46) is related structurally to carbon dioxide, CO2. Draw a picture showing the orbitals involved in the and bonds CO2, and identify the likely hybridization of carbon.
-
Almost all stable organic species have tetravalent carbon atoms, but species with trivalent carbon atoms also exist. Carbocations are one such class of compounds. (a) How many valence electrons do...
-
JetPak, Inc., delivers overnight business letters internationally and very recently began building a new capital investment in an airport distribution center in Cincinnati. The project has been...
-
The following information about the payroll for the week ended December 30 was obtained from the records of Saine Co.: Salaries: Sales salaries Deductions: $180,000 Income tax withheld $65,296...
-
You have just been hired as the chief executive officer (CEO) in a medium-sized organization. The organization is not suffering financially, but neither is it doing as well as it could do. This is...
-
The following is the selling price and cost information about three joint products: X Y Z Anticipated production 1 2 , 0 0 0 lbs . 8 , 0 0 0 lbs . 7 , 0 0 0 lbs . Selling price / lb . at split - off...
-
calculate the maximum bending compressive stress of the following section under NEGATIVE bending moment of 216KN.m. 216mm 416mm 316mm 115mm
-
Need assistance with the following forms: 1040 Schedule 1 Schedule 2 Schedule C Schedule SE Form 4562 Form 8995 Appendix B, CP B-3 Christian Everland (SS number 412-34-5670) is single and resides at...
-
Which statement is inappropriate? People will participate and add to a CRSA workshop if they are . . . . . . ..: a. committed to the workshop objective b. have something of value to add c. believe...
-
Given that all the choices are true, which one concludes the paragraph with a precise and detailed description that relates to the main topic of the essay? A. NO CHANGE B. Decades, X-ray C. Decades...
-
The vant Hoff corollary to the third law of thermodynamics is that whenever two solid forms of a substance are known, the one with the greater specific heat will be the more stable one at higher...
-
Draw structure for the neutral molecule represented by the following model. Explain whether the octet rule is satisfied at each atom of the compound. Draw all of the important resonance structures...
-
Indicate whether each of these species can act as an acid, a base, or both: H +1 a) H-N-H 1 H H e) H-C-O-H H b) H-O-H H: H TI f) H-C-C-C-H H H c) H-C-H H H d):Ci : g) H-O-C-:
-
Show the conjugate acids of these species: a) CH-O-H b) H-O: C) CH,NH,
-
X Your answer is incorrect. Flounder Consulting Corp. company records revealed the following for the current year: What was the net cash flow from operating activities for the year? $ 0 $ 9 8 0 0...
-
Assume that interest rate parity holds. The U.S. fiveyear interest rate is 0.08 annualized, and the Mexican fiveyear interest rate is 0.05 annualized. Todays spot rate of the Mexican peso is $0.21....
-
find the NSP of a whole life insurance.6 with $100,000 Death benefits, for a female aged 105 years, if i=10%? (use Australian life Tables 2005-07) find the NSP of a whole life insurance.6 with...

Study smarter with the SolutionInn App


