Identify all nonbonding lone pairs of electron in the following molecules, and tell what geometry you expect
Question:
Identify all nonbonding lone pairs of electron in the following molecules, and tell what geometry you expect for each pf the indicated atoms.
(a) The oxygen atom in the dimethyl ether, CH3 ? O ? CH3
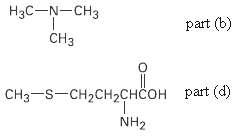
(b) The nitrogen atom in trim ethylamine,
(c) The phosphorus atom in the Phosphine, PH3
(d) The sulfur atom in the amino acid methionine,
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





