Question: Identify the indicated hydrogen?s in the following molecules as pro-R or pro-S: (a) (b) .co2 H3N Alanine (S)-Glyceraldehyde
Identify the indicated hydrogen?s in the following molecules as pro-R or pro-S:
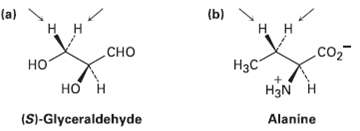
(a) (b) .co2 H3N Alanine (S)-Glyceraldehyde
Step by Step Solution
3.48 Rating (164 Votes )
There are 3 Steps involved in it
Strategy For each molecule replace the left hydrogen with 2H Give priorities to ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
22-C-O-S (140).docx
120 KBs Word File


