Arrange these compounds in order of increasing acid strength: CH3COH .. HOC-COH HOCCH2CH2COH
Question:
Arrange these compounds in order of increasing acid strength:
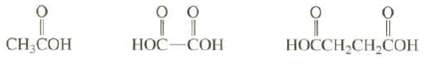
Transcribed Image Text:
。 CH3COH 오오.. 오 HOC-COH HOCCH2CH2COH
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (18 reviews)
CH COH weake...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Arrange these compounds in order of increasing SN2 reaction rate: CI Br CI Br
-
Arrange these compounds in order of increasing acid strength: CHOH CN CN
-
Arrange the following compounds in order of increasing boiling point. Explain your answer in terms of the intermolecular forces in each compound. (a) (b) (c) (d)
-
Under what circumstance is it most appropriate to travel in reverse with a load? A. When you are carrying a non-standard load B. When a tall load obstructs your forward vision C. When the load is...
-
You have just been appointed the cost accountant for a new company called Amazing Joy Flights. In its first few years the company will provide three types of scenic flights: 'Over and Around...
-
Pam Corporation paid $960,000 cash for a 100 percent interest in Sun Corporation on January 1, 2017, when Sun's stockholders' equity consisted of $400,000 capital stock and $160,000 retained...
-
3. Pops inventory at December 31, 2017, included items acquired from Son on which Son made a profit of $1,200,000. Total sales by Son to Pop during 2017 were $4,800,000.
-
Coffee Palaces manager, Joe Felan, suspects that demand for mocha latte coffees depends on the price being charged. Based on historical observations, Joe has gathered the following data, which show...
-
PLEASE PROVIDE LINK TO EXCEL SHEET You are trying to decide how much to save for retirement. Assume you plan to save $ 6 , 5 0 0 per year with the first investment made one year from now. You think...
-
Allie has bought a new apple orchard. The orchard has a single file of trees, numbered from 1 to N. Each tree has a certail number of ripe apples. Allie has a rule she wants to follow. She wants to...
-
Arrange these compounds in order of increasing base strength: NH NH NH NO 9 CH,CNH,
-
Use the tables in this chapter to predict whether these equilibria favor the reactants or the products: CHH CH + CHCH-N-CHCH, NHCHC=C: + :NH c) CHC=C-H+ (CH3)3C-0: Dc0: CH3 CH3 + CHCH,CHCH, +...
-
Refer to Exercise 19.25. Use the Tukey-Kramer W procedure to compare the different geographic areas by chain means. Use a 5 .05. In Exercise 19.25 The marketing research group of a corporation...
-
Construct a 90% confidence interval for the population standard deviation o at Bank B. Bank B 4.2 5.4 5.9 6.1 6.6 7.7 7.7 8.6 9.3 10.0
-
Jamila Traders has a head office in Nanyuki and an autonomous branch in Thika. The trial balances of the head office and the branch as at 30 September 2014 were as follows: Head office Sh. Sh. Thika...
-
Poll Results in the Media USA Today provided results from a survey of 1144 Americans who were asked if they approve of Brett Kavanaugh as the choice for Supreme Court justice. 51% of the respondents...
-
ROI analysis using the DuPont model a. Firm A has a margin of 7%, sales of $980,000, and ROI of 19.6%. Calculate the firm's average total assets. b. Firm B has net income of $259,200, turnover of...
-
The test statistic of z = - 2.93 is obtained when testing the claim that p < 2/ 3. This is a left-tailed test. Using a 0.01 significance level, complete parts (a) and (b). a. Find the critical...
-
How many hats did you recognize being worn in Case Study 4.1? I recognized at least 15. (See Appendix C for these.)
-
Explain how two samples can have the same mean but different standard deviations. Draw a bar graph that shows the two samples, their means an standard deviations as error bars. T S
-
Prove that a b lal 101'
-
Paraldehyde, a sedative and hypnotic agent, is prepared by treatment of acetaldehyde with an acidic catalyst. Propose a mechanism for the reaction. . C H+ H catalyst C Paraldehyde
-
The Meerwein?Ponndorf?Verley reaction involves reduction of a ketone by treatment with an excess of aluminum triisopropoxide. The mechanism the process is closely related to the Cannizzaro reaction...
-
Propose a mechanism to account for the formation of 3, 5-dimethylpyrazole from hydrazine and 2, 4-pcntancdionc. Look carefully to see what has happened to each carbonyl carbon in going from starting...
-
Product Weight Sales Additional Processing Costs P 300,000 lbs. $ 245,000 $ 200,000 Q 100,000 lbs. 30,000 -0- R 100,000 lbs. 175,000 100,000 If joint costs are allocated based on relative weight of...
-
The projected benefit obligation was $380 million at the beginning of the year. Service cost for the year was $21 million. At the end of the year, pension benefits paid by the trustee were $17...
-
CVP Modeling project The purpose of this project is to give you experience creating a multiproduct profitability analysis that can be used to determine the effects of changing business conditions on...

Study smarter with the SolutionInn App


