Arrange these compounds in order of increasing acid strength: CHOH CN CN
Question:
Arrange these compounds in order of increasing acid strength:
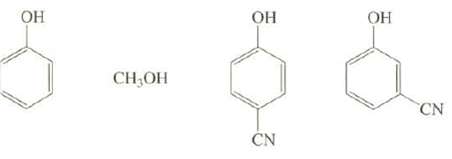
Transcribed Image Text:
ОН CH₂OH ОН CN ОН CN
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 63% (11 reviews)
The acidic hydrogen is the one bonded to the oxygen for each compound Compound B is a str...View the full answer

Answered By

Tobias sifuna
I am an individual who possesses a unique set of skills and qualities that make me well-suited for content and academic writing. I have a strong writing ability, allowing me to communicate ideas and arguments in a clear, concise, and effective manner. My writing is backed by extensive research skills, enabling me to gather information from credible sources to support my arguments. I also have critical thinking skills, which allow me to analyze information, draw informed conclusions, and present my arguments in a logical and convincing manner. Additionally, I have an eye for detail and the ability to carefully proofread my work, ensuring that it is free of errors and that all sources are properly cited. Time management skills are another key strength that allow me to meet deadlines and prioritize tasks effectively. Communication skills, including the ability to collaborate with others, including editors, peer reviewers, and subject matter experts, are also important qualities that I have. I am also adaptable, capable of writing on a variety of topics and adjusting my writing style and tone to meet the needs of different audiences and projects. Lastly, I am driven by a passion for writing, which continually drives me to improve my skills and produce high-quality work.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Arrange these compounds in order of increasing acid strength: CH3COH .. HOC-COH HOCCH2CH2COH
-
Arrange these compounds in order of increasing SN2 reaction rate: CI Br CI Br
-
Arrange the following compounds in order of increasing boiling point. Explain your answer in terms of the intermolecular forces in each compound. (a) (b) (c) (d)
-
To encourage _________, leaders are encouraged to study the principles of teamwork that prevail in clinical contexts for their application to managerial planning and decision-making contexts....
-
During January, Microchem Ltd produced 1000 units of special product called Stylex, and the counting records indicated the following: Direct material purchased .................. 36 0000 kilograms @...
-
The stockholders' equities of Pam Corporation and its 80 percent-owned subsidiary, Sun Corporation, on December 31, 2016, are as follows (in thousands): Pam's Investment in Sun account balance on...
-
1. Son was a 75 percentowned subsidiary of Pop Corporation throughout the 20162018 period. Pops separate income (excludes income from Son) was $21,600,000, $20,400,000, and $24,000,000 in 2016, 2017,...
-
Briefly discuss the interrelated components that should exist within an internal control system. In your opinion, which component is the most important and why?
-
You are trying to estimate the value of a property that you are interested in buying. The subject property is located at 3 2 2 Rock Creek Road in a new suburb of a large metropolitan area. The...
-
What are some of the competitive advantages Vivobarefoot gained through its infrastructure update? One ongoing concern for Vivobarefoot is the quality and speed of the Internet service available to...
-
Explain which species is the stronger base: a) :CH or :CH NO b) CH-P-CH, or CH-N-CH CH3 CH3 : : c) BICH,CO: or CICH,CO: d) CHO: or CHNH
-
Arrange these compounds in order of increasing base strength: NH NH NH NO 9 CH,CNH,
-
The article "Three Sisters Give Birth on the Same Day" (Chance, Spring 2001, 23-25) used the fact that three Utah sisters had all given birth on March 11, 1998 as a basis for posing some interesting...
-
Write a brief statement that interprets the confidence interval. Choose the correct answer below. A. There is a 99% chance that the true value of the population mean weight of newborn girls will fall...
-
Transcribed image text: If estimated annual factory overhead is $1,072,500; overhead is applied using direct labor hours, estimated annual direct labor hours are 275,000 actum March factory overhead...
-
Your firm has limited capital to invest and is therefore interested in comparing projects based on the profitability index (PI), as well as other measures. What is the PI of the project with the...
-
The following rates are applicable to annual payroll in British Columbia Question 17 options: 1234 1.95% x total B.C. remuneration 1234 2.925% x (B.C. remuneration - $500,000) 1234 Tax Rate 1234...
-
Assume that different groups of couples use a particular method of gender selection and each couple gives birth to one baby. This method is designed to increase the likelihood that each baby will be...
-
Internal Auditors as Facilitators of Risk Management Synopsis Describes how Control and Risk Self Assessment has been implemented in BT plc, facilitated by internal auditing developing a toolkit...
-
What mass of H2 will be produced when 122 g of Zn are reacted? Zn(s) + 2HCl(aq) ( ZnCl2(aq) + H2(g)
-
Prove formula (e) of Theorem 3 using mathematical induction. Data from Formula e of theorem 3 (e) ;3 i=1 n(n + 1) 2
-
Each of the following reaction schemes contains one or more flaws. What is wrong in each case? How would you correct eachscheme? (a) Ag*, NH,OH 1. CH3MgBr 2. * CH , I b) H H2 CH * CHgCHHIO)2 (c) ...
-
6-Methyl-5-hepten-2-one is a constituent of lemongrass oil. How could you synthesize this substance from methyl4-oxopentanoate? CH3CH2CH2OCH3 Methyl 4-oxopentanoate
-
Aldehydes and ketones react with thiols to yield thioacetals just as they react with alcohols to yield acetals. Predict the product of the following reaction, and propose a mechanism: H* catalyst 2...
-
1. (A nice inharitage) Suppose $1 were invested in 1776 at 3.3% interest compounded yearly a) Approximatelly how much would that investment be worth today: $1,000, $10,000, $100,000, or $1,000,000?...
-
Why Should not the government subsidize home buyers who make less than $120K per year. please explain this statement
-
Entries for equity investments: 20%50% ownership On January 6, 20Y8, Bulldog Co. purchased 25% of the outstanding common stock of $159,000. Gator Co. paid total dividends of $20,700 to all...

Study smarter with the SolutionInn App


