Use the tables in this chapter to predict whether these equilibria favor the reactants or the products:
Question:
Use the tables in this chapter to predict whether these equilibria favor the reactants or the products:
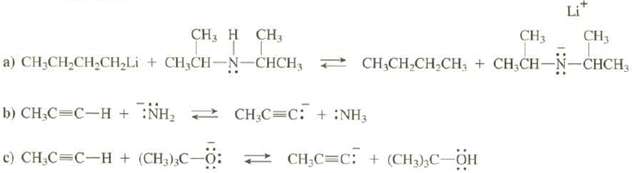
Transcribed Image Text:
CH₂H CH₂ + CHỊCH-N-CHCH, NH₂CH₂C=C: + :NH₂ c) CH₂C=C-H+ (CH3)3C-0: Dc0: CH3 CH3 + CHỊCH,CHỊCH, + CHỊCH–N–CHCH, a) CHỊCH,CH,CH,Li b) CH₂C=C-H+ CH₂C=C: + (CH₂)₂C-OH
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 69% (13 reviews)
a The products are favored because butyl lithium is a stron...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
The information in many of the tables in this chapter can be found in the Economic Report of the President, which appears annually. Using a recent issue of the report at your library or on the...
-
Use the data visualization methods presented in this chapter to explore these data and discover relationships between the variables. Include the following, in your report: 1. Create a scatter chart...
-
Use the social security model developed in this chapter to answer this question. Suppose that the government establishes a social security program in period T, which provides a social security...
-
1. Consider the market for local rides (taxis, Uber Lyft, and so on), which is highly competitive. Suppose that the market is initially unregulated, but that the government imposes a binding price...
-
The accountant for Lane and Company uses a statistical control chart to help management to decide when to investigate variances. The critical value is one standard deviation from the mean. The...
-
Pop Corporation paid $3,000,000 for an 80 percent interest in Son Corporation on January 1, 2016, when the book values and fair values of Son's assets and liabilities were as follows (in thousands):...
-
4. There were no unrealized profits in the December 31, 2018, inventories of either company.
-
The Effect of Transactions on the Accounting Equation For each of the following transactions, indicate whether it increases (I), decreases (D), or has no effect (NE) on the total dollar amount of...
-
Blackstone is contemplating a leveraged buyout of MGM Mirage. MGM ' s 1 . 2 billion shares currently trade at $ 1 1 ? share, and the company has $ 1 2 billion in long - term debt, $ 2 billion in...
-
Mohan is a sole trader who does not maintain a full set of accounting records. He was able to provide the following information: Fixtures should be depreciated by 10% per annum on the cost of...
-
Arrange these compounds in order of increasing acid strength: CH3COH .. HOC-COH HOCCH2CH2COH
-
Complete these equilibrium reaction sin the most reasonable manner possible using the curved arrow convention to show the movement of electrons in the reactions, Predict whether the reactants or the...
-
Compute MACRS depreciation for the following qualified assets for the calendar years 2019 and 2020: (Ignore bonus depreciation and the Section 179 deduction.) Asset Business equipment (7-year...
-
The pulse rates of 152 randomly selected adult males vary from a low of 37 bpm to a high of 117 bpm. Find the minimum sample size required to estimate the mean pulse rate of adult males. Assume that...
-
A rod 12.0 cm long is uniformly charged and has a total charge of -23.0 C. Determine the magnitude and direction of the electric field along the axis of the rod at a point 32.0 cm from its center....
-
Hello need help with this problem. The transactions relating to the formation of Blue Company Stores Incorporated, and its first month of operations follow. a. The firm was organized and the...
-
At the beginning of the year, the net assets of Shannon Company were $492,600. The only transactions affecting stockholders equity during the year were net income of $70,200 and dividends of $15,400....
-
The claim is that smokers have a mean cotinine level greater than the level of 2.84 ng/mL found for nonsmokers. (Cotinine is used as a biomarker for exposure to nicotine.) The sample size is n = 739...
-
What were the four issues in internal auditing, recognized by Albrecht et al. in 1992 and discussed in the chapter, and how have these influenced the wearing of hats by internal auditors in your...
-
Nitrogen monoxide reacts with hydrogen as follows: 2NO(g)+ H2(g) N2O(g) + H2O(g) The rate law is [H2]/ t = k[NO]2[H2], where k is 1.10 107 L2/(mol2s) at 826oC. A vessel contains NO and H2 at...
-
Solve the inequality. |x 4| < 1
-
In light of ?our answer to Problem 19.56, propose a mechanism for the formation of 3, 5 -dimethylisoxazole from hydroxylamine and 2, 4-pentanedione. CH 3,5-Dimethylisoxazole
-
Tran?s alkenes are converted into their cis isomers and vice versa on epoxidation followed by treatment of the epoxide with triphenylphosphine. Propose a mechanism for the epoxide ? alkene reaction. ...
-
Treatment of an ?, ? unsaturated ketone with basic aqueous hydrogen peroxide yields an epoxy ketone. The reaction is specific to unsaturated ketones; isolated alkene double bonds do not react....
-
A government bond matures in 30 years, makes semi-annual coupon payments of 6.0% ($120 per year) and offers a yield of 3.7% annually compounded. Assume face value is $1,000. Three years later the...
-
Your objective is: 1. Carry out a life insurance needs analysis, for each one of them (show your calculations) [30 Marks] 2. Refer to the case and the insurance plan quotes. Would you recommend...
-
TufStuff, Incorporated, sells a wide range of drums, bins, boxes, and other containers that are used in the chemical industry. One of the company s products is a heavy - duty corrosion - resistant...

Study smarter with the SolutionInn App


