Benzyl bromide is converted into benzaldehyde by heating in dimethyl sulfoxide. Propose a structure for the intermediate,
Question:
Benzyl bromide is converted into benzaldehyde by heating in dimethyl sulfoxide. Propose a structure for the intermediate, and show the mechanisms of the two steps in thereaction.
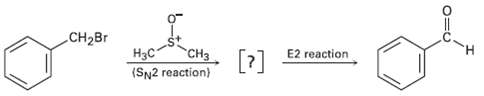
Transcribed Image Text:
CH2BR E2 reaction [?] Нас CH3 (SN2 reaction)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 45% (11 reviews)
HCSCH3 0 CH CH325 SN2 displacement occurs when the n...View the full answer

Answered By

Dorcas Juliet
I am a proficient tutor and writer with over 4 years experience, I can deliver A+ works in all fields related to business and economics subject. Kindly hire me for excellent papers
4.70+
10+ Reviews
51+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Benzyl chloride can be converted into benzaldehyde by treatment with nitro methane and base. The reaction involves initial conversion of nitro methane into its anion, followed by SN2 reaction of the...
-
Propose a structure for an alcohol with molecular formula C5H12 O that has the 1H NMR spectrum given in Fig. 9.46. Assign the chemical shifts and splitting patterns to specific aspects of the...
-
Propose a structure for ether with molecular formula C 7 H 8 O that exhibits the following 13 C NMR spectrum. Carbon NMR 114.0 129.5, 120,714.0 55.1- 159.71 160 120 140 100 100 Chemical shift (ppm)...
-
Which sets of lines can be removed without stopping the code from compiling and while printing the same output? (Choose three.) A. Lines 15 and 17 B. Lines 16 and 23 C. Lines 17, 18, and 22 D. Line...
-
During the year, Tamara had capital transactions resulting in gains (losses) as follows: Sold stock in ABC Company (acquired two years ago).........................($1,500) Sold collectible coins...
-
Analysis of variance and multiple regression have many similarities. Which of the following is not true? a. The response variable is quantitative for each. b. They both have F tests for testing that...
-
Which of the following statements are true of long-term investments? a. They are held as an investment of cash available for current operations. b. They can include funds earmarked for a special...
-
On January 1, 2014, Abraham Company purchased the following two machines for use in its production process. Machine A: The cash price of this machine was $55,000. Related expenditures included: sales...
-
Tolent, a local HR Consulting firm, has total partners' equity of $792,000, which is made up of Hall, Capital. $616,000, and Reynolds, Capital, $176,000. The partners share profittosses) in a ratio...
-
Matilda Crone opened a public relations firm called Dance Fever on August 1, 2014. The following amounts summarize her business on August 31, 2014: During September 2014, the business completed the...
-
You know the mechanism of HBr addition to alkenes, and you know the effects of various substituent groups on aromatic substitution. Use this knowledge to predict which of the following two alkenes...
-
Use your knowledge of directing effects, along with the following data, to deduce the directions of the dipole moments in aniline andBromobenzene. Br- -NH2 Br- -NH2 H = 1.52 D H = 2.91 D A= 1.53 D
-
The sucrose substitute tagatose is produced by hydrolyzing lactose and then chemically converting one of the two resulting aldoses to a ketose. Which residue of lactose gives rise to tagatose?
-
Sketch plane / intersecting plane K. Then draw a line & in plane J that intersects plane Kat a single point. A X C B D E
-
Use a graphing utility to verify any five of the graphs that you drew by hand in Exercises 126. Data from exercise 1-26 1. x + 2y = 8 3. x2y> 10 2. 3x6y 12 4. 2xy > 4
-
The following information pertains to Porter Company for 2011. Ending inventory consisted of 30 units. Porter sold 320 units at \(\$ 30\) each. All purchases and sales were made with cash. Required...
-
In Problems 7780, use a numerical integration routine on a graphing calculator to find the area bounded by the graphs of the indicated equations over the given interval (when stated). Compute answers...
-
Solar Heating, Inc., had the following transactions for 2011: Required a. Determine the quantity and dollar amount of inventory at the end of the year, assuming Solar Heating Inc. uses the FIFO cost...
-
HOW DOES THE INTERNET WORK?
-
The vapor pressure of the liquid NH, is measured at different temperatures. The following vapor pressure data are obtained. Temperature, K P, mmHg 217.1 223.4 234.7 588.1 Calculate the enthalpy of...
-
Using a T-S diagram, discuss the effect of subcooling in the condenser and superheating in the evaporator on the efficiency of a Rankine (or other) power generation cycle.
-
Explain which compound has the higher melting point or boiling point: a) Melting point b) Boiling point or C COCH c) Boiling point or
-
What is the functional group present in these compounds? SCH3 SH C CH, - Ph b) ) H3 f) CH,CH,SOH d) CH,CH,SPh e) CH,SCH3
-
Identify the most acidic site in thesecompounds: NH2 CH.COCH,CH,CH3 c) a) b) CH3 e) CH;CH,CH,COH d) CH,CH,CCH,CH3
-
Comparative financial statements for Weller Corporation, a merchandising company, for the year ending December 31 appear below. The company did not issue any new common stock during the year. A total...
-
Mrquered Mrquered
-
You plan to invest $10,00 today in an investment account earning 5% interest. You then plan to invest an additional $1,000 into this account each year for the next twenty years. How much money will...

Study smarter with the SolutionInn App


