Benzyl chloride can be converted into benzaldehyde by treatment with nitro methane and base. The reaction involves
Question:
Benzyl chloride can be converted into benzaldehyde by treatment with nitro methane and base. The reaction involves initial conversion of nitro methane into its anion, followed by SN2 reaction of the anion with benzyl chloride and subsequent E2 reaction. Write the mechanism in detail, using curved arrows to indicate the electron flow in each step.
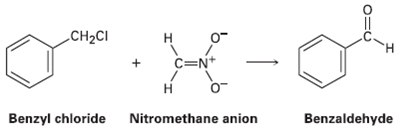
Transcribed Image Text:
CH2CI н C=N+ Н Benzyl chloride Nitromethane anion Benzaldehyde
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (10 reviews)
09100 CH 0 ...View the full answer

Answered By

Carly Cimino
As a tutor, my focus is to help communicate and break down difficult concepts in a way that allows students greater accessibility and comprehension to their course material. I love helping others develop a sense of personal confidence and curiosity, and I'm looking forward to the chance to interact and work with you professionally and better your academic grades.
4.30+
12+ Reviews
21+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Using curved arrows to indicate the election flow in each step, show how the base-catalyzed reverse aldol reaction of 4-hydroxy-4-methly-2-pentanone takes place to yield 2 equivalents of acetone.
-
Benzyl bromide is converted into benzaldehyde by heating in dimethyl sulfoxide. Propose a structure for the intermediate, and show the mechanisms of the two steps in thereaction. CH2BR E2 reaction...
-
Show how 4-methylcyclohexyl chloride can be converted to 4- methylcyclohexanecarboxylic acid.
-
IFRS requires the following: (a) Ending inventory is written up and down to net realizable value each reporting period. (b) Ending inventory is written down to net realizable value but cannot be...
-
The model below depicts industry analysis as a funnel that focuses on remote-factor analysis to better understand the impact of factors in the operating environment. Do you find this model...
-
Use the frequency polygon to identify the class with the greatest, and the class with the least, frequency. MCAT Scores for 90 Applicants 16 14 12 10- 6. 472 480 488 496 504 512 520 528 Score...
-
16. Construct a spreadsheet that permits you to compute payoff and profit for a short and long stock, a short and long forward, and purchased and written puts and calls.
-
Consider the following key performance indicators and classify each indicator according to the balanced scorecard perspective it addresses. Choose from the financial perspective, customer...
-
CISC 3415 HW Assignment - 5 (4pts) 1. (2-pt) The picture in Figure 1 (which should be familiar from Project 5) is a plan of an area that you have to program a robot to navigate in. At different...
-
Print-for-All is a family-owned print shop that has grown from a three-press two-color operation to a full-service facility capable of performing a range of jobs from simple copying to four-color...
-
Rank the following substituted phenols in order of increasing acidity, and explain your answer: CH
-
Reduction of 2-butanone with NaBH4 yields 2-butanol. Is the product chiral is it optically active? Explain.
-
In 1965, Intel cofounder Gordon Moore initiated what has since become known as Moores law: The number of transistors per square inch on integrated circuits will double approximately every 18 months....
-
The following information summarizes the activities in the Mixing Department for the month of March. Beginning inventory 1 , 0 0 0 units, 8 0 % complete Started and completed 2 4 , 5 0 0 units Ending...
-
What is your recommendation for the maximum size of coarse aggregate for the following situation? A continuously reinforced concrete pavement cross section contains a layer of No. 6 reinforced bars...
-
On January 1, 2024, Winn Heat Transfer leased office space under a three-year operating lease agreement. The arrangement specified three annual lease payments of $72,000 each, beginning December 31,...
-
A closed square pyramid tank (base width: 6.0 m; height 3.0 m), sitting on its square base, has a 1.0 m depth of water. Suppose this tank is inverted (turned upside down) and is made to stand on its...
-
P.4.3 Apply a Taylor series expansion to a mixed backward formula for the first derivative: (Ux)i = 1 Ax (aui-2+ bui-1 + cu + dui+1) Derive the family of second order accurate formulas and the...
-
In a normal distribution, what z-score value separates the lowest 20% of the distribution from the highest 80%? a. z 5 0.20 b. z 5 0.80 c. z 5 0.84 d. z 5 20.84
-
Refer to the information from Exercise 22-19. Use the information to determine the (1) Weighted average contribution margin , (2) Break-even point in units, and (3) Number of units of each product...
-
The enthalpy of formation of NaI(s) is -288 kJ mol -1 . Use this value, together with other data in the text, to calculate the lattice energy of NaI(s).
-
List the following compounds in order of decreasing reactivity toward CH3O- in an SN2 reaction carried out in CH3OH: CH3F, CH3Cl, CH3Br, CH3I, CH3OSO2CF3, 14CH3OH.
-
Starting with (S)-2-bromobutane, outline syntheses of each of the following compounds: (a) (b) (c) (d) (R)-CH3CHCH2CH3 OCH2CH3 (R)-CH3CHCH2CH3 CCH3 (R)-CH3CHCH2CH3 SH (R)-CH3CHCH2CH3 SCH3
-
Which alkyl halide would you expect to react more rapidly by an SN2 mechanism? Explain your answer. (a) (b) (c) (d) (e) Br r or or CI
-
7 . 4 3 Buy - side vs . sell - side analysts' earnings forecasts. Refer to the Financial Analysts Journal ( July / August 2 0 0 8 ) study of earnings forecasts of buy - side and sell - side analysts,...
-
Bond P is a premium bond with a coupon of 8.6 percent , a YTM of 7.35 percent, and 15 years to maturity. Bond D is a discount bond with a coupon of 8.6 percent, a YTM of 10.35 percent, and also 15...
-
QUESTION 2 (25 MARKS) The draft financial statements of Sirius Bhd, Vega Bhd, Rigel Bhd and Capella for the year ended 31 December 2018 are as follows: Statement of Profit or Loss for the year ended...

Study smarter with the SolutionInn App


