Calculate the pKa for these compounds. a) HCOH (K 1.75 X 104) b) CHCH3 (K = 10-50)
Question:
Calculate the pKa for these compounds.
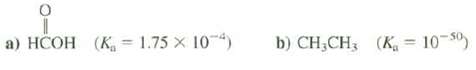
Transcribed Image Text:
a) HCOH (K 1.75 X 104) b) CH₂CH3 (K = 10-50)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (12 reviews)
p K a...View the full answer

Answered By

Ali Khawaja
my expertise are as follows: financial accounting : - journal entries - financial statements including balance sheet, profit & loss account, cash flow statement & statement of changes in equity -consolidated statement of financial position. -ratio analysis -depreciation methods -accounting concepts -understanding and application of all international financial reporting standards (ifrs) -international accounting standards (ias) -etc business analysis : -business strategy -strategic choices -business processes -e-business -e-marketing -project management -finance -hrm financial management : -project appraisal -capital budgeting -net present value (npv) -internal rate of return (irr) -net present value(npv) -payback period -strategic position -strategic choices -information technology -project management -finance -human resource management auditing: -internal audit -external audit -substantive procedures -analytic procedures -designing and assessment of internal controls -developing the flow charts & data flow diagrams -audit reports -engagement letter -materiality economics: -micro -macro -game theory -econometric -mathematical application in economics -empirical macroeconomics -international trade -international political economy -monetary theory and policy -public economics ,business law, and all regarding commerce
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Using the pKa values of analogous compounds in Table 3.1, predict which would be the stronger base. (a) (b) (c) (d) or (CHa),Cor O: HO or
-
Compounds like CCl2F2 are known as chlorofluorocarbons, or CFCs. These compounds were once widely used as refrigerants but are now being replaced by compounds that are believed to be less harmful to...
-
The pKa of protonated acetone is about -7.5 and the pKa of protonated hydroxylamine is 6.0. a. In its reaction with hydroxylamine at pH = 4.5 (Figure 18.2), what fraction of acetone will be present...
-
Q1-Mutual funds provide the following for their shareholders. A. diversification B. professional management C. record keeping and administration D. all of these options
-
Office Magic Ltd manufactures ergonomic office equipment. The standard cost for material and labour is $190 per unit. This includes 8 kilograms of direct material at a standard cost of $5 per...
-
Do policies reflect political ideologies or public demands? Argue your position with examples.
-
How would you describe the ideal candidate?
-
Calculating Internal Growth the most recent financial statements for Benatar Co. are shown here: Assets and costs are proportional to sales. Debt and equity are not. The company maintains a constant...
-
XYZ Industries manufactures 21,000 components per year. The total manufacturing costs for this level of production were determined as follows: Direct Materials Direct Labor Variable manufacturing...
-
Refer to the HR Report section of the Inquirer. Baldwin spends $485,492 on various HR initiatives. What percentage of this expenditure is dedicated to training its employees? Select: 1 58.2% 64.7%...
-
Which of these species can behave as a Lewis base? a) CH0CH,CH, CH3 d) CH3NH + b) CH3CHCH3 0: I.. e) CHCOH c) CH3NH
-
Calculate the Ka for these compounds. a) HC=CH (pK = 25) b) HC N (pK = 9.31)
-
Use the data given in Short Exercise 8-3. Assume that in November 2013, Western Motors sold half its investment in Phase Motors. The sale price was $130 million. Compute Western Motors gain or loss...
-
Carol's Cupcakes has grown from a home business into a one of the largest event and wedding catering companies in the area. Its founder, Carol Thompson, first dreamed of owning her own company while...
-
Many things have changed for businesses in 2022. The previous 2 business years of 2020 and 2021 have tested businesses and the workforce like nothing else. Not only were profits reduced, and...
-
1) Virginia Tech's motto is "Ut Prosim" which means 'That I May Serve'. Share how you contribute to a community that is important to you. How long have you been involved? What have you learned and...
-
Person Is Arianna Grande Answer all questions Who are they? How successful are they? Why would companies be interested in partnering with them? Identify one company from their industry that you feel...
-
Imagine you have just retired after a long and very successful career (as a physiotherapist). Congratulations! You've made such an impact in the world that business and community leaders from around...
-
Explain the framework of organisational justice in the business? LO1
-
a. What is the cost of borrowing if Amarjit borrows $28 500 and repays it over a four-year period? b. How many shares of each stock would he get if he used the $28 500 and invested equally in all...
-
Prove the identity. sin(/2 + x) = cos x
-
Show how the Wittig reaction might be used to prepare the following alkenes. Identify the alkyl halide and the carbonyl components that would he used. (b) (a)
-
How would you use a Grignard reaction on an aldehyde or ketone to synthesize the following compounds? (a) 2-Pentanol (b) 1-Butanol (c) 1-Phenylcyclohexanol (d) Diphenyl methanol
-
Aldehydes can be prepared by the Wittig reaction using (methoxymethylene)-triphenylphosphorane as the Wittig reagent and then hydrolyzing the product with acid. For example, (a) How would you prepare...
-
(15 points) Stressed $2.500,000 of S% 20 year bands. These bonds were issued Jary 1, 2017 and pay interest annually on each January 1. The bonds yield 3% and was issued at $325 8S! Required (2)...
-
Packaging Solutions Corporation manufactures and sells a wide variety of packaging products. Performance reports are prepared monthly for each department. The planning budget and flexible budget for...
-
1. A company issued 10%, 10-year bonds with a par value of $1,000,000 on January 1, at a selling price of $885,295 when the annual market interest rate was 12%. The company uses the effective...

Study smarter with the SolutionInn App


