Question: Carbocations, ions that contain a trivalent, positively charged carbon atom, react with water to give alcohols. How can you account for the fact that the
Carbocations, ions that contain a trivalent, positively charged carbon atom, react with water to give alcohols. How can you account for the fact that the following carbocation gives a mixture of two alcohols on reaction withwater?
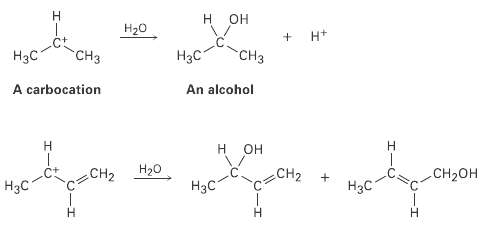
2 + "CH "CH H A carbocation An alcohol CH2 CH2 CH- H3C
Step by Step Solution
★★★★★
3.30 Rating (165 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock

The cation pictured can be represented ... View full answer

Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock
Document Format (1 attachment)
22-C-O-A-B-R (104).docx
120 KBs Word File


