Discuss the stability of these structures: a) H H-C-O-H H b) H H-N H H
Question:
Discuss the stability of these structures:
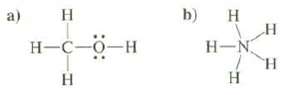
Transcribed Image Text:
a) H H-C-O-H H b) H H-N H H
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 58% (17 reviews)
The octet rule is the most im...View the full answer

Answered By

Somshukla Chakraborty
I have a teaching experience of more than 4 years by now in diverse subjects like History,Geography,Political Science,Sociology,Business Enterprise,Economics,Environmental Management etc.I teach students from classes 9-12 and undergraduate students.I boards I handle are IB,IGCSE, state boards,ICSE, CBSE.I am passionate about teaching.Full satisfaction of the students is my main goal.
I have completed my graduation and master's in history from Jadavpur University Kolkata,India in 2012 and I have completed my B.Ed from the same University in 2013. I have taught in a reputed school of Kolkata (subjects-History,Geography,Civics,Political Science) from 2014-2016.I worked as a guest lecturer of history in a college of Kolkata for 2 years teaching students of 1st ,2nd and 3rd year. I taught Ancient and Modern Indian history there.I have taught in another school in Mohali,Punjab teaching students from classes 9-12.Presently I am working as an online tutor with concept tutors,Bangalore,India(Carve Niche Pvt.Ltd.) for the last 1year and also have been appointed as an online history tutor by Course Hero(California,U.S) and Vidyalai.com(Chennai,India).
4.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Discuss the stability of each of these species based on the octet rule and formal charges; . b) H- a) e) -N d) H- -N-C e)
-
Discuss the stability of (a) A submerged, (b) A floating body whose center of gravity is above the center of buoyancy.
-
Rank the stability of these resonance structures? H-CH a) CH=CH-CH=CH b) :0: CH-CH=CH-CH :0: 12+
-
A spring is at rest in the vertical direction. When a 5 kg mass is placed upon the spring, the length of the spring compresses to 0 . 2 0 meters. The 5 kg mass is removed and replaced by an 8 kg...
-
Refer to the data given in Problem 15.35 for Pally Communications Company prior to the JIT purchasing agreement. The lead time required to receive an order of XL-20 is one month. Required: 1....
-
Chris Guthrie was recently hired by S&S Air, Inc., to assist the company with its financial planning, and to evaluate the company's performance. Chris graduated from college five years ago with a...
-
Compare and contrast an operating lease with a capital lease. AppendixLO1
-
Look back at Section 2.3 and then answer the following questions: a. The price of Estee Lauder stock has risen to $90. What is the market value of the firm's equity? b. The rating agency has revised...
-
A physical inventory count of Garden Company has a $74,250 balance before considering the following: Consigned goods sent to and held by Madison Company: $13,225 Inventory in transit sold FOB...
-
Rose-comb chickens mated with walnut-comb chickens produced 15 walnut-, 14 rose-, 5 pea-, and 6 single-comb chicks. Determine the genotypes of the parents.
-
Show a Lewis structure for the simplest neutral compound formed from hydrogen and sulfur.
-
Write Lewis structures for these compounds: (a) C 3 H 8 (b) C 2 H 2 (c) CH 3 N (d) NH 3 O
-
Analyze equity section of balance sheet. (LO 1,5) PetsMart reported the following information on the financial statements included with its 2004 annual report. Were any new shares of common stock...
-
The Tokyo Olympics. After watching how the tokyo olympics became the most expensive summer game ever video answer the following questions. Q 3 : As you saw in the video, the capital investment a city...
-
write at least two paragraphs discussing the experiences of individuals who identify outside the traditional binary gender system (male/female.) Please explore the challenges they face and how...
-
Newly formed S&J Iron Corporation has 163,000 shares of $5 par common stock authorized. On March 1, Year 1, S&J Iron issued 9,000 shares of the stock for $12 per share. On May 2, the company issued...
-
Use the SMOKE for this question. The variable cigs is the number of cigarettes smoked per day. How many people in the sample do not smoke at all? What fraction of people claim to smoke 20 cigarettes...
-
Transcribed image text : Reproduced below from Farthington Supply's accounting records is the accounts receivable subledger along with selected general ledger accounts. Dec. 31/19 Balance Credit...
-
Distinguish between work in process, finished goods, and cost of goods sold.
-
Record the following selected transactions for March in a two-column journal, identifying each entry by letter: (a) Received $10,000 from Shirley Knowles, owner. (b) Purchased equipment for $35,000,...
-
Sketch the graph of the function y = ln(x 2) 1.
-
Following is a molecule model of aspirin (acetylsalicylic acid). Identify the hydribization of each carbon atom in aspirin, and tell which atoms have lone pairs of electrons (gray = c. red = O, ivory...
-
Draw a line-bond structure for the propyne, CH3C CH; indicate the hydribization of each carbon; and predict a value for each bond angle.
-
Identify all nonbonding lone pairs of electron in the following molecules, and tell what geometry you expect for each pf the indicated atoms. (a) The oxygen atom in the dimethyl ether, CH3 ? O ? CH3...
-
Date Account and Explanation Debit Credit Jun 1 Cash 105,000 Jun 1 Capital 105,000 (capital contribution) Jun 1 Computer Equipment 56,000 Jun 1 Cash 56,000 Jun 1 Cash 198,000 Jun 1 Bank Loan Payable...
-
.Is bankruptcy on the part of the borrower a common risk that frequently interferes with a lenders efforts to work out a defaulted loan through either nonforeclosure means or foreclosure? Discuss.
-
For each of the following, compute the future value: Present Value Years Interest Rate $ 1 , 2 5 0 1 9 1 2 % $ 9 8 , 7 2 7 1 5 1 3 % $ 6 2 5 6 1 2 % $ 1 1 7 , 6 2 2 7 1 6 % 2 . For each of the...

Study smarter with the SolutionInn App


