Draw the product from reaction of each of the following substances with (i) Br2, FeBr3 and (ii)
Question:
Draw the product from reaction of each of the following substances with (i) Br2, FeBr3 and (ii) CH3COCl,AlCl3.
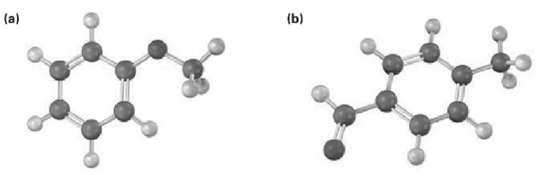
Transcribed Image Text:
(b) (a)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 53% (15 reviews)
a The methoxyl group is an orthopara director OCH3 OCH3 Br FeBr3 Br pBromome...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Give the major product obtained from reaction of each of the following with excess HCl: a. CH3CH2C==CH b. CH3CH2C===CCH2CH3 c. CH3CH2C==CCH2CH2CH3
-
Predict the product from reaction of the following substance (reddish brown = Br) with: (a) PBr3 (b) Aqueous H2SO4 (c) SOCl2 (d) PCC (e) Br2,FeBr3
-
Predict the product from reaction of the following substance with: (a) NaBH4 then H3O+ (b) LiAlH4 then H3O+ (c) CH3CH2 MgBr; then H3O+
-
What is the probability that the number X of successes in the personnel managers sample of 100 employees in question 10 will be 48 or more?
-
In reporting the transactions of a self-employed taxpayer, when can a Schedule C-EZ be used instead of the regular Schedule C of Form 1040?
-
No express warranties should be created by the oral statements made by salespersons about a product. Debate this
-
For each of the following situations, identify (1) the case as either (a) a present or a future value and (b) a single amount or an annuity, (2) the table you would use in your computations (but do...
-
Label each of the following accounts as an asset (A), liability (L), owners equity (OE), revenue (R), or expense (E). Indicate the financial statement on which the account belongsincome statement...
-
Do in 5 mins please Calculate Variable Overhead Variances from the following information : Budgeted Activity Actual Activity Actual Production Actual Variable Overhead Budget of Variable Overhead...
-
For a construction job, a contractor can haul rock by means of an ordinary 10 cubic yard dump truck, or he can use a combination rig where a 10 cubic yard also pulls an 8 cubic yard trailer. When...
-
In planning synthesis, it?s as important to know what not do as to know what to do. As written, the following reaction schemes have flaws in them. What is wrong with each? (a) CN CN 1. CH3CH,COCI,...
-
The following molecular model of a dimethyl-substituted biphenyl represents the lowest-energy conformation of the molecule. Why are the two benzene rings tilted at a 63 o angle to each other rather...
-
Table 11.3 (page 509) identifies three risks typically encountered when determining product production requirements. Required (a) Analyse the degree of exposure to each of these risks for the...
-
Sketch the requested conic sections in Problems 14-23 using the definition. A parabola with the distance between the directrix and focus 1 unit
-
Why is the Rosenblum case a particularly important case in auditor liability?
-
Draw a population curve for a city whose growth rate is \(1.3 \%\) and whose present population is 53,000 . The equation is \[P=P_{0} e^{r t}\] Let \(t=0,10, \cdots, 50\) to help you find points for...
-
Draw a bar graph for each data set in Problems 32-35. Data set B Data set A: The annual wages of employees at a small accounting firm are given in thousands of dollars. 35 25 25 16 14 1 2 25 18 2 2...
-
For the four unrelated situations, A-D, below, calculate the unknown amounts indicated by the letters appearing in each column: B D Beginning Assets... Liabilities.. $40,000 $12,000 $28,000 $ (d)...
-
What other applications are there for cities using Miovisions smart intersection products?
-
Uniform electric field in Figure a uniform electric field is directed out of the page within a circular region of radius R = 3.00 cm. The magnitude of the electric field is given by E = (4.50 x 10-3...
-
A tank containing carbon dioxide at 400 K and 50 bar is vented until the temperature in the tank falls to 300 K. Assuming there is no heat transfer between the gas and the tank, find the pressure in...
-
Explain why only one of the two chlorines of 1, 2-dichloro-2-methylpropane is replaced by a hydroxy group when the compound is heated in water (see the preceding hydrolysis reaction.
-
On the basis of the bond cleavage shown for this reaction in Figure 10.1, predict the stereo chemistry of the product.Explain. OCCH, CH,CH, ." -
-
Show the products of thesereactions: CI CH3CO, NaOH a) DMSO . Br CH,CO, b) DMF CH
-
Choose two stocks from the same industry to minimize the influence of other confounding factors. You choose the industry that you are relatively more familiar with, and then estimate the implied...
-
why should Undertake research to review reasons for previous profit or loss?
-
A pension fund's liabilities has a PV01 of $200 million. The plan has $100 billion of assets with a weighted average modified duration of 8. The highest duration bond that the plan can invest in has...

Study smarter with the SolutionInn App


