Evidence for restricted rotation around amide CO?N bonds comes from NMR studies. At room temperature, the 1
Question:
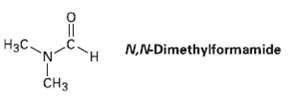
Evidence for restricted rotation around amide CO?N bonds comes from NMR studies. At room temperature, the 1H NMR spectrum of N, N-dimethyl form amide shows three peaks: 2.9 ? (singlet, 3 H), 3.0 ? (singlet, 3 H), 8.0 ? (singlet, 1 H). As the temperature is raised, however, the two singlets at 2.9 ? and 3.0 ? slowly merge. At 180 ?C, the 1H NMR spectrum shows only two peaks: 2.95 ? (singlet, 6 H) and 8.0 ? (singlet, 1 H). Explain this temperature-dependent behavior.
Transcribed Image Text:
N,N-Dimethylformamide Нзс. CHз
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (16 reviews)
H NMR shows that the two methyl groups of NNdimethylformamide are nonequ...View the full answer

Answered By

Ali Khawaja
my expertise are as follows: financial accounting : - journal entries - financial statements including balance sheet, profit & loss account, cash flow statement & statement of changes in equity -consolidated statement of financial position. -ratio analysis -depreciation methods -accounting concepts -understanding and application of all international financial reporting standards (ifrs) -international accounting standards (ias) -etc business analysis : -business strategy -strategic choices -business processes -e-business -e-marketing -project management -finance -hrm financial management : -project appraisal -capital budgeting -net present value (npv) -internal rate of return (irr) -net present value(npv) -payback period -strategic position -strategic choices -information technology -project management -finance -human resource management auditing: -internal audit -external audit -substantive procedures -analytic procedures -designing and assessment of internal controls -developing the flow charts & data flow diagrams -audit reports -engagement letter -materiality economics: -micro -macro -game theory -econometric -mathematical application in economics -empirical macroeconomics -international trade -international political economy -monetary theory and policy -public economics ,business law, and all regarding commerce
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
The 1H NMR spectrum of compound A (C8H8O) consists of two singlets of equal area at 5.1 (sharp) and 7.2 ppm (broad). On treatment with excess hydrogen bromide, compound A is converted to a single...
-
The 1H NMR spectrum of N,N-dimethylformamide shows a separate signal for each of the two methyl groups. Can you explain why?
-
The 1H NMR spectrum of the product obtained when an unknown alkene reacts with ozone and the ozonolysis product is worked up under oxidizing conditions is shown. Identify the alkene. 10 (ppm)...
-
Suppose a consumer lives in two periods , with his income in period 1 as $100 and his income in period 2 as $150. If the rate of interest in the economy is 12%. Find the equilibrium level of...
-
In what three situations should you consider using passive voice?
-
Verify the Cayley-Hamilton Theorem for A = The Cayley-Hamilton Theorem can be used to calculate powers and inverses of matrices. For example, if A is a 2 Ã 2 matrix with characteristic...
-
7. Examine the grid outline of the PMBOK Guide in Figure CE19-3 (page 570). a. Explain, in general terms, how each of the knowledge areas in that grid pertains to information systems projects. b....
-
Linkin Corporation is considering purchasing a new delivery truck. The truck has many advantages over the company's current truck (not the least of which is that it runs). The new truck would cost...
-
Dyckman Dealers has an investment in Thomas Corporation bonds that Dyckman accounts for as a trading security. Thomas Corporation's bonds are publicly traded and the prevailing market price indicates...
-
Paque Corporation owns 90% of the common stock of Segal Company. The stock was purchased for $810,000 on January 1, 2009, when Segal Companys retained earnings were $150,000. Financial data for 2013...
-
Cytochrome c is an enzyme found in the cells of all aerobic organisms. Elemental analysis of cytochrorne c shows that it contains 0.43% iron. What is the minimum molecular weight of this enzyme?
-
Propose a structure for an octapeptide that shows the composition Asp, Gly2 Leu, Phe, Pro2, Val on amino acid analysis. Edman analysis shows a glycine N-terminal group, and leucine is the C-terminal...
-
DielsAlder cycloaddition of 1, 3-butadiene with acrylonitrile requires that the diene be in a cis (or cis-like) conformation: In fact, the diene exists primarily in a trans conformation, the cis...
-
Identify at least two business systems that support the development of effective work relationships Briefly explain how each system supports the development of effective work relationships.
-
Power and Influence Personal Plan - How will you navigate the realms of power and influence? Why is this personal plan important for you? What do you want to achieve? do a table with SMART goals -...
-
A single-stage trickling-filter plant is proposed for treating a dilute wastewater with a BOD concentration of 170 mg/L. The plant is located in a warm climate, and the minimum wastewater temperature...
-
For the first assignment for this course, compose a written document that contains the following: A description and assessment of your past experiences with policy and program planning, either your...
-
What are the key motivators driving consumer purchasing decisions in our industry? How do consumers perceive our brand compared to competitors, and what factors influence brand loyalty?
-
Define NoSQL data store and give three examples. Explain how NoSQL will likely be used in organizations and state why learning Microsoft Access is still important to you. Explain what is unusual...
-
Perform the indicated operations. In designing a cam for a fire engine pump, the expression is used. Simplify this expression. (3) (3 4 32
-
Caffeine (C 8 H 10 N 4 O 2 ) is a weak base with a pKb of 10.4. Calculate the pH of a solution containing a caffeine concentration of 455 mg/L.
-
Give a synthesis of from aniline. NHCH2CHs
-
Give a synthesis for from toluene. H2N CH3 NH2
-
Show how CH3CH2N(CH3)2 can be synthesized from an amide.
-
QUESTION 3 A business owns seven flats rented out to staff at R500 per month. All flats were tenanted Ist january 21 months rent was in arrears and as at 31st December 14 months' rent wa Identify the...
-
1. 2. 3. Select the Tables sheet, select cells A6:B10, and create range names using the Create from Selection button [Formulas tab, Defined Names group]. Select cells B1:F2 and click the Name box....
-
Tropical Rainwear issues 3,000 shares of its $18 par value preferred stock for cash at $20 per share. Record the issuance of the preferred shares. (If no entry is required for a particular...

Study smarter with the SolutionInn App


