How would you prepare the following alkyl halides from the correspondingalcohols? CH (a) CI (b) Br CH
Question:
How would you prepare the following alkyl halides from the correspondingalcohols?
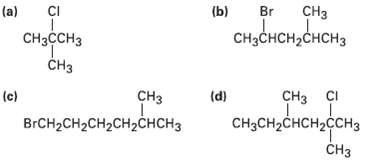
Transcribed Image Text:
CHз (a) CI (b) Br CнзссHз CHзснсH2CнсHз CHз (c) CI сHз снз (d) BrCH2CH2CH2CHгснCHз CHзCH2снсH2cсH3 CHз
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (10 reviews)
Remember that halogen acids are used for converting tertiary alcohols to al...View the full answer

Answered By

Marcus Solomon
I am committed to ensuring that my services always meet the clients' expectations.
4.60+
82+ Reviews
117+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Which of the following alkyl halides form a substitution product in an SN1 reaction that is different from the substitution product formed in an SN2 reaction? a. b. c. d. e. f. CH Br CH CHCHCHCH CHa...
-
How would you prepare the following substances from Cyclopentanol? More than one step may be required. (a) Cyclopentanone (b) Cyclopentane (c) 1-Methylcyclopentanol (d) Trans-2-Methylcyclopentanol I...
-
How would you prepare the following compound using an acetoacetic estersynthesis?
-
Tiffee Company identifies the following items for possible inclusion in the physical inventory. Indicate whether each item should be included or excluded from the inventory taking. (a) 900 units of...
-
Consider the example of the new product at Curtis Distributors, which was analyzed using Holt's method in Figure 7.12. Repeat the forecasts using one smaller value of a than the value of 0.2 used in...
-
Water at the rate of 118 ft 3 /min flows through a smooth horizontal tube 250 ft long. The pressure drop is 4.55 psi. Determine the tube diameter.
-
Summarize how consumers evaluate extensions and how extensions contribute to parent brand equity. LO3
-
Sebastian and Whitley, plumbers, successfully bid $30,000 for the plumbing work on a new luxury home. Total direct labor cost on the job was $9,500, other direct costs were $2,500, and overhead is...
-
Discuss the concept of duration
-
During two recent years Perez Construction, Inc., disposed of the following plant and equipment: Required: 1. Determine the cash flow from the sale of property for each year that would be reported in...
-
What products would you expect from reaction of the following alkenes with NBS? If more than one product is formed, show the structures ofall. H (a) . (b) CH3D2H
-
How strong a base would you expect a Grignard reagent to be? Look at Table 8.1, and then predict whether the following reactions will occur as written.?(The p K a of NH 3 is 35.) (a) CH 3 MgBr + H ?...
-
In Problems 13 26, find the zeros of each quadratic function by factoring. What are the x-intercepts of the graph of the function? f (x) = x( x 4) 12
-
6.10 Long Div. and Comp Square Calculus - No Calculator Find the indefinite integral. 1. S 4x-34x+56x-21 4x-2 dx Mastery Check #2 1 dx 2. Sx-4x+5x x-4x+5 S S Name: Sienna Nono Date: 3-1-24 Period:...
-
How well are oncology firms leveraging digital technology to gain and sustain competitive advantage?
-
Axel and Brooklyn have agreed to buy a new vehicle. Brooklyn mentions that she is looking forward to getting a new SUV, so they have room for their dogs and kids. Axel mentions he thought they were...
-
Discuss how technology and human resources are needed to operate this facility in this behind the scenes look at this retailing giant. Support your opinion with research and/or key concepts covered...
-
4.2 At a given instant, a spacecraft is 500 km above the earth, with a right ascension of 300 and a declination of -20 relative to the geocentric equatorial frame. Its velocity is 10 km/s directly...
-
Explain why orientation should precede job training.
-
For each of the following reactions, express the equilibrium constant: a) H20 (I) H2 (g) + 02 (g) Ke = 1.0x107 b) Fe2 (g) 2F (g) Ke= 4.9 x 10-21 c) C (s) + O2 (g) d) H2 (g) + C2H4 (g) C2H6 (g) Ke =...
-
Write a molecular equation for the gas-evolution reaction that occurs when you mix aqueous nitric acid and aqueous sodium carbonate.
-
The cyclohexane chair just drawn has the headrest to the left and the footrest to the right. Draw a cyclohexane chair with its axial and equatorial bonds, having the headrest to the right and the...
-
Draw 1, 2, 3, 4, 5, 6-hexamethylcyclohexane with all the methyl groups (a) in axial positions. (b) in equatorial positions. If your cyclohexane rings look awkward or slanted when using the analytical...
-
Draw a Newman projection, similar to Figure 3-25, down the bond in the equatorial conformation of methylcyclohexane. Show that the equatorial methyl group is also anti to C5. (Using your models will...
-
Questien It Calraluta bae neark yoe cen atforal to berren
-
In calculating the net present value of a proposed project, the cash flows of the project should include a.) amortization of goodwill b.) interest expenses paid to bondholders c.) extra working...
-
If Yolanda's insurance company cancels her fire insurance policy after 204 days, how much of the $682.00 annual premium will she receive as a refund (in $)? (Round you answer to the nearest cent.) $

Study smarter with the SolutionInn App


